Human Monoclonal Antibody 81.39a Effectively Neutralizes Emerging Influenza A Viruses of Group 1 and 2 Hemagglutinins
- PMID: 27630240
- PMCID: PMC5110155
- DOI: 10.1128/JVI.01284-16
Human Monoclonal Antibody 81.39a Effectively Neutralizes Emerging Influenza A Viruses of Group 1 and 2 Hemagglutinins
Abstract
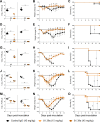
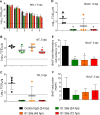
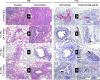
The pandemic threat posed by emerging zoonotic influenza A viruses necessitates development of antiviral agents effective against various antigenic subtypes. Human monoclonal antibody (hMAb) _targeting the hemagglutinin (HA) stalk offers a promising approach to control influenza virus infections. Here, we investigated the ability of the hMAb 81.39a to inhibit in vitro replication of human and zoonotic viruses, representing 16 HA subtypes. The majority of viruses were effectively neutralized by 81.39a at a 50% effective concentration (EC50) of <0.01 to 4.9 μg/ml. Among group 2 HA viruses tested, a single A(H7N9) virus was not neutralized at 50 μg/ml; it contained HA2-Asp19Gly, an amino acid position previously associated with resistance to neutralization by the group 2 HA-neutralizing MAb CR8020. Notably, among group 1 HA viruses, H11-H13 and H16 subtypes were not neutralized at 50 μg/ml; they shared the substitution HA2-Asp19Asn/Ala. Conversely, H9 viruses harboring HA2-Asp19Ala were fully susceptible to neutralization. Therefore, amino acid variance at HA2-Asp19 has subtype-specific adverse effects on in vitro neutralization. Mice given a single injection (15 or 45 mg/kg of body weight) at 24 or 48 h after infection with recently emerged A(H5N2), A(H5N8), A(H6N1), or A(H7N9) viruses were protected from mortality and showed drastically reduced lung viral titers. Furthermore, 81.39a protected mice infected with A(H7N9) harboring HA2-Asp19Gly, although the antiviral effect was lessened. A(H1N1)pdm09-infected ferrets receiving a single dose (25 mg/kg) had reduced viral titers and showed less lung tissue injury, despite 24- to 72-h-delayed treatment. Taken together, this study provides experimental evidence for the therapeutic potential of 81.39a against diverse influenza A viruses.
Importance: Zoonotic influenza viruses, such as A(H5N1) and A(H7N9) subtypes, have caused severe disease and deaths in humans, raising public health concerns. Development of novel anti-influenza therapeutics with a broad spectrum of activity against various subtypes is necessary to mitigate disease severity. Here, we demonstrate that the hemagglutinin (HA) stalk-_targeting human monoclonal antibody 81.39a effectively neutralized the majority of influenza A viruses tested, representing 16 HA subtypes. Furthermore, delayed treatment with 81.39a significantly suppressed virus replication in the lungs, prevented dramatic body weight loss, and increased survival rates of mice infected with A(H5Nx), A(H6N1), or A(H7N9) viruses. When tested in ferrets, delayed 81.39a treatment reduced viral titers, particularly in the lower respiratory tract, and substantially alleviated disease symptoms associated with severe A(H1N1)pdm09 influenza. Collectively, our data demonstrated the effectiveness of 81.39a against both seasonal and emerging influenza A viruses.
Copyright © 2016 Marjuki et al.
Figures
Similar articles
-
Potential Role of Nonneutralizing IgA Antibodies in Cross-Protective Immunity against Influenza A Viruses of Multiple Hemagglutinin Subtypes.J Virol. 2020 Jun 1;94(12):e00408-20. doi: 10.1128/JVI.00408-20. Print 2020 Jun 1. J Virol. 2020. PMID: 32269119 Free PMC article.
-
Hemagglutinin stalk-based monoclonal antibody elicits broadly reactivity against group 1 influenza A virus.Virol J. 2020 Dec 7;17(1):191. doi: 10.1186/s12985-020-01458-z. Virol J. 2020. PMID: 33287849 Free PMC article.
-
Cross-protective potential of a novel monoclonal antibody directed against antigenic site B of the hemagglutinin of influenza A viruses.PLoS Pathog. 2009 Mar;5(3):e1000350. doi: 10.1371/journal.ppat.1000350. Epub 2009 Mar 20. PLoS Pathog. 2009. PMID: 19300497 Free PMC article.
-
One step closer to universal influenza epitopes.Expert Rev Anti Infect Ther. 2009 Aug;7(6):687-90. doi: 10.1586/eri.09.48. Expert Rev Anti Infect Ther. 2009. PMID: 19681695 Review.
-
Tackling influenza with broadly neutralizing antibodies.Curr Opin Virol. 2017 Jun;24:60-69. doi: 10.1016/j.coviro.2017.03.002. Epub 2017 May 18. Curr Opin Virol. 2017. PMID: 28527859 Free PMC article. Review.
Cited by
-
Post-transplant Viral Respiratory Infections in the Older Patient: Epidemiology, Diagnosis, and Management.Drugs Aging. 2017 Oct;34(10):743-754. doi: 10.1007/s40266-017-0491-5. Drugs Aging. 2017. PMID: 28965331 Free PMC article. Review.
-
Influenza virus glycoprotein-reactive human monoclonal antibodies.Microbes Infect. 2020 Jul-Aug;22(6-7):263-271. doi: 10.1016/j.micinf.2020.06.003. Epub 2020 Jun 19. Microbes Infect. 2020. PMID: 32569735 Free PMC article. Review.
-
Evolution of Therapeutic Antibodies, Influenza Virus Biology, Influenza, and Influenza Immunotherapy.Biomed Res Int. 2018 May 28;2018:9747549. doi: 10.1155/2018/9747549. eCollection 2018. Biomed Res Int. 2018. PMID: 29998138 Free PMC article. Review.
-
H7N9 avian influenza A virus in China: a short report on its circulation, drug resistant mutants and novel antiviral drugs.Expert Rev Anti Infect Ther. 2017 Aug;15(8):723-727. doi: 10.1080/14787210.2017.1353419. Epub 2017 Jul 17. Expert Rev Anti Infect Ther. 2017. PMID: 28692316 Free PMC article.
-
Universal influenza virus vaccines and therapeutic antibodies.Clin Microbiol Infect. 2017 Apr;23(4):222-228. doi: 10.1016/j.cmi.2017.02.009. Epub 2017 Feb 12. Clin Microbiol Infect. 2017. PMID: 28216325 Free PMC article. Review.
References
-
- Marty FM, Man CY, van der Horst C, Francois B, Garot D, Manez R, Thamlikitkul V, Lorente JA, Alvarez-Lerma F, Brealey D, Zhao HH, Weller S, Yates PJ, Peppercorn AF. 2014. Safety and pharmacokinetics of intravenous zanamivir treatment in hospitalized adults with influenza: an open-label, multicenter, single-arm, phase II study. J Infect Dis 209:542–550. doi:10.1093/infdis/jit467. - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention. 2006. High levels of adamantane resistance among influenza A (H3N2) viruses and interim guidelines for use of antiviral agents–United States, 2005-06 influenza season. MMWR Morb Mortal Wkly Rep 55:44–46. - PubMed
-
- Baranovich T, Bahl J, Marathe BM, Culhane M, Stigger-Rosser E, Darnell D, Kaplan BS, Lowe JF, Webby RJ, Govorkova EA. 2015. Influenza A viruses of swine circulating in the United States during 2009-2014 are susceptible to neuraminidase inhibitors but show lineage-dependent resistance to adamantanes. Antiviral Res 117:10–19. doi:10.1016/j.antiviral.2015.02.004. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

