Effect of 8-hydroxyquinoline and derivatives on human neuroblastoma SH-SY5Y cells under high glucose
- PMID: 27635352
- PMCID: PMC5012261
- DOI: 10.7717/peerj.2389
Effect of 8-hydroxyquinoline and derivatives on human neuroblastoma SH-SY5Y cells under high glucose
Abstract
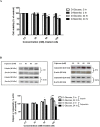
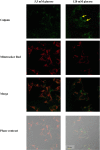
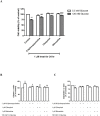
8-Hydroxyquinoline and derivatives exhibit multifunctional properties, including antioxidant, antineurodegenerative, anticancer, anti-inflammatory and antidiabetic activities. In biological systems, elevation of intracellular calcium can cause calpain activation, leading to cell death. Here, the effect of 8-hydroxyquinoline and derivatives (5-chloro-7-iodo-8-hydroxyquinoline or clioquinol and 8-hydroxy-5-nitroquinoline or nitroxoline) on calpain-dependent (calpain-calpastatin) pathways in human neuroblastoma (SH-SY5Y) cells was investigated. 8-Hydroxyquinoline and derivatives ameliorated high glucose toxicity in SH-SY5Y cells. The investigated compounds, particularly clioquinol, attenuated the increased expression of calpain, even under high-glucose conditions. 8-Hydroxyquinoline and derivatives thus adversely affected the promotion of neuronal cell death by high glucose via the calpain-calpastatin signaling pathways. These findings support the beneficial effects of 8-hydroxyquinolines for further therapeutic development.
Keywords: 8-Hydroxyquinoline; Calpain; Clioquinol; High glucose; Neuronal cells; Nitroxoline.
Conflict of interest statement
The authors declare there are no competing interests.
Figures
Similar articles
-
The protective effect of melatonin on methamphetamine-induced calpain-dependent death pathway in human neuroblastoma SH-SY5Y cultured cells.J Pineal Res. 2010 Mar;48(2):94-101. doi: 10.1111/j.1600-079X.2009.00731.x. Epub 2009 Dec 30. J Pineal Res. 2010. PMID: 20050990
-
Calpastatin overexpression reduces oxidative stress-induced mitochondrial impairment and cell death in human neuroblastoma SH-SY5Y cells by decreasing calpain and calcineurin activation, induction of mitochondrial fission and destruction of mitochondrial fusion.Mitochondrion. 2016 Sep;30:151-61. doi: 10.1016/j.mito.2016.07.009. Epub 2016 Jul 21. Mitochondrion. 2016. PMID: 27453331
-
Trichostatin A epigenetically increases calpastatin expression and inhibits calpain activity and calcium-induced SH-SY5Y neuronal cell toxicity.FEBS J. 2013 Dec;280(24):6691-701. doi: 10.1111/febs.12572. Epub 2013 Nov 7. FEBS J. 2013. PMID: 24200051
-
Neuroprotective effects of Mycoplasma hyorhinis against amyloid-β-peptide toxicity in SH-SY5Y human neuroblastoma cells are mediated by calpastatin upregulation in the mycoplasma-infected cells.Neurochem Int. 2011 Mar;58(4):497-503. doi: 10.1016/j.neuint.2011.01.005. Epub 2011 Jan 8. Neurochem Int. 2011. PMID: 21219955
-
8-Hydroxyquinolines in medicinal chemistry: A structural perspective.Eur J Med Chem. 2016 Sep 14;120:252-74. doi: 10.1016/j.ejmech.2016.05.007. Epub 2016 May 6. Eur J Med Chem. 2016. PMID: 27191619 Review.
Cited by
-
Cellulose Acetate-Based Electrospun Materials with a Variety of Biological Potentials: Antibacterial, Antifungal and Anticancer.Polymers (Basel). 2021 May 18;13(10):1631. doi: 10.3390/polym13101631. Polymers (Basel). 2021. PMID: 34069809 Free PMC article.
-
New insights into the mechanism of antifungal action of 8-hydroxyquinolines.Saudi Pharm J. 2019 Jan;27(1):41-48. doi: 10.1016/j.jsps.2018.07.017. Epub 2018 Jul 20. Saudi Pharm J. 2019. PMID: 30662305 Free PMC article.
-
(8-Hydroxyquinoline) Gallium(III) Complex with High Antineoplastic Efficacy for Treating Colon Cancer via Multiple Mechanisms.ACS Omega. 2023 Feb 8;8(7):6945-6958. doi: 10.1021/acsomega.2c07742. eCollection 2023 Feb 21. ACS Omega. 2023. PMID: 36844596 Free PMC article.
-
Integrative proteomic and functional analyses provide novel insights into the action of the repurposed drug candidate nitroxoline in AsPC-1 cells.Sci Rep. 2020 Feb 13;10(1):2574. doi: 10.1038/s41598-020-59492-4. Sci Rep. 2020. PMID: 32054977 Free PMC article.
-
α-Glucosidase and α-Amylase Inhibitory Potentials of Quinoline-1,3,4-oxadiazole Conjugates Bearing 1,2,3-Triazole with Antioxidant Activity, Kinetic Studies, and Computational Validation.Pharmaceuticals (Basel). 2022 Aug 22;15(8):1035. doi: 10.3390/ph15081035. Pharmaceuticals (Basel). 2022. PMID: 36015183 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

