MiR-182 promotes proliferation and invasion and elevates the HIF-1α-VEGF-A axis in breast cancer cells by _targeting FBXW7
- PMID: 27648365
- PMCID: PMC5004079
MiR-182 promotes proliferation and invasion and elevates the HIF-1α-VEGF-A axis in breast cancer cells by _targeting FBXW7
Abstract
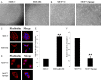
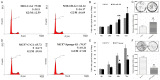
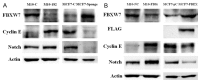
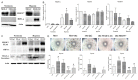
The feature of imperfect complementary effect of miRNAs to mRNAs implies that miRNAs may simultaneously _target different mRNAs to affect multiple aspects of tumorigenesis. In our previous results, we demonstrated that miR-182 was over-expressed in breast cancer cell lines and clinical tumor tissues and its up-regulation increased tumorigenicity and invasiveness by repressing a tumor suppressor RECK. In this study, we showed that overexpression miR-182 regulated actin distribution and filopodia formation to increase invasiveness of breast cancer cells. In addition, miR-182 enhanced cell cycle progression and proliferation. We further identified the E3 ubiquitin-protein ligase FBXW7 as a _target gene of miR-182. We also demonstrated that miR-182-overexpressing cells were highly sensitive to hypoxia. Under hypoxic condition, HIF-1α and VEGF-A proteins were significantly upregulated in these cells. In addition, the conditioned medium of miR-182-overexpressing cells contained more VEGF-A than the control cells and induced angiogenesis more efficiently in vitro. All these effects could be counteracted by ectopic expression of FBXW7 in cells or neutralization of VEGF-A in the conditioned media by specific antibody. Finally, our data showed that miR-182 expression was inversely correlated with FBXW7 in breast tumor tissues. In conclusion, our study explores a novel mechanism by which miR-182 elevates HIF-1α expression to promote breast cancer progression.
Keywords: FBXW7; HIF-1α; MiR-182; VEGF-A; breast cancer.
Figures

Similar articles
-
miR-497 suppresses angiogenesis in breast carcinoma by _targeting HIF-1α.Oncol Rep. 2016 Mar;35(3):1696-702. doi: 10.3892/or.2015.4529. Epub 2015 Dec 29. Oncol Rep. 2016. PMID: 26718330
-
Hypoxia-inducible microRNA-224 promotes the cell growth, migration and invasion by directly _targeting RASSF8 in gastric cancer.Mol Cancer. 2017 Feb 7;16(1):35. doi: 10.1186/s12943-017-0603-1. Mol Cancer. 2017. PMID: 28173803 Free PMC article.
-
PRL-3 promotes gastric cancer migration and invasion through a NF-κB-HIF-1α-miR-210 axis.J Mol Med (Berl). 2016 Apr;94(4):401-15. doi: 10.1007/s00109-015-1350-7. Epub 2015 Nov 9. J Mol Med (Berl). 2016. PMID: 26548949
-
miR-92a is upregulated in cervical cancer and promotes cell proliferation and invasion by _targeting FBXW7.Biochem Biophys Res Commun. 2015 Feb 27;458(1):63-9. doi: 10.1016/j.bbrc.2015.01.066. Epub 2015 Jan 24. Biochem Biophys Res Commun. 2015. PMID: 25623537
-
MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells.Cell Oncol (Dordr). 2017 Oct;40(5):457-470. doi: 10.1007/s13402-017-0335-7. Epub 2017 Jul 24. Cell Oncol (Dordr). 2017. PMID: 28741069
Cited by
-
Clinical significance of FBXW7 loss of function in human cancers.Mol Cancer. 2022 Mar 26;21(1):87. doi: 10.1186/s12943-022-01548-2. Mol Cancer. 2022. PMID: 35346215 Free PMC article. Review.
-
Potential utility of miRNAs for liquid biopsy in breast cancer.Front Oncol. 2022 Aug 4;12:940314. doi: 10.3389/fonc.2022.940314. eCollection 2022. Front Oncol. 2022. PMID: 35992785 Free PMC article. Review.
-
miRNA expression in advanced Algerian breast cancer tissues.PLoS One. 2020 Feb 10;15(2):e0227928. doi: 10.1371/journal.pone.0227928. eCollection 2020. PLoS One. 2020. PMID: 32040529 Free PMC article.
-
Molecular insights and clinical implications for the tumor suppressor role of SCFFBXW7 E3 ubiquitin ligase.Biochim Biophys Acta Rev Cancer. 2024 Sep;1879(5):189140. doi: 10.1016/j.bbcan.2024.189140. Epub 2024 Jun 21. Biochim Biophys Acta Rev Cancer. 2024. PMID: 38909632 Review.
-
FBXW7 attenuates tumor drug resistance and enhances the efficacy of immunotherapy.Front Oncol. 2023 Mar 14;13:1147239. doi: 10.3389/fonc.2023.1147239. eCollection 2023. Front Oncol. 2023. PMID: 36998461 Free PMC article. Review.
References
-
- Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. - PubMed
-
- Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6:376–385. - PubMed
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
-
- Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132:4653–4662. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
