Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a
- PMID: 27670592
- PMCID: PMC5068234
- DOI: 10.1084/jem.20160258
Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a
Abstract
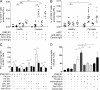
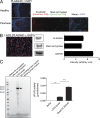
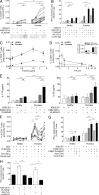
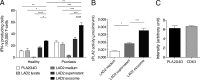
Psoriasis is a chronic inflammatory skin disease associated with a T helper 17 response. Yet, it has proved challenging to identify relevant peptide-based T cell antigens. Antigen-presenting Langerhans cells show a differential migration phenotype in psoriatic lesions and express constitutively high levels of CD1a, which presents lipid antigens to T cells. In addition, phospholipase A2 (PLA2) is highly expressed in psoriatic lesions and is known to generate neolipid skin antigens for recognition by CD1a-reactive T cells. In this study, we observed expression of a cytoplasmic PLA2 (PLA2G4D) in psoriatic mast cells but, unexpectedly, also found PLA2G4D activity to be extracellular. This was explained by IFN-α-induced mast cell release of exosomes, which transferred cytoplasmic PLA2 activity to neighboring CD1a-expressing cells. This led to the generation of neolipid antigens and subsequent recognition by lipid-specific CD1a-reactive T cells inducing production of IL-22 and IL-17A. Circulating and skin-derived T cells from patients with psoriasis showed elevated PLA2G4D responsiveness compared with healthy controls. Overall, these data present an alternative model of psoriasis pathogenesis in which lipid-specific CD1a-reactive T cells contribute to psoriatic inflammation. The findings suggest that PLA2 inhibition or CD1a blockade may have therapeutic potential for psoriasis.
© 2016 Cheung et al.
Figures


Similar articles
-
Filaggrin inhibits generation of CD1a neolipid antigens by house dust mite-derived phospholipase.Sci Transl Med. 2016 Feb 10;8(325):325ra18. doi: 10.1126/scitranslmed.aad6833. Sci Transl Med. 2016. PMID: 26865566 Free PMC article.
-
Phospholipase activity of acyloxyacyl hydrolase induces IL-22-producing CD1a-autoreactive T cells in individuals with psoriasis.Eur J Immunol. 2022 Mar;52(3):511-524. doi: 10.1002/eji.202149485. Epub 2022 Jan 10. Eur J Immunol. 2022. PMID: 34913478 Free PMC article.
-
CD1a presentation of endogenous antigens by group 2 innate lymphoid cells.Sci Immunol. 2017 Dec 22;2(18):eaan5918. doi: 10.1126/sciimmunol.aan5918. Sci Immunol. 2017. PMID: 29273672 Free PMC article.
-
CD1a and skin T cells: a pathway for therapeutic intervention.Clin Exp Dermatol. 2024 Apr 23;49(5):450-458. doi: 10.1093/ced/llad460. Clin Exp Dermatol. 2024. PMID: 38173286 Free PMC article. Review.
-
Activation of human T cells by CD1 and self-lipids.Immunol Rev. 2015 Sep;267(1):16-29. doi: 10.1111/imr.12322. Immunol Rev. 2015. PMID: 26284469 Free PMC article. Review.
Cited by
-
Current Understanding of the Roles of CD1a-Restricted T Cells in the Immune System.Mol Cells. 2021 May 31;44(5):310-317. doi: 10.14348/molcells.2021.0059. Mol Cells. 2021. PMID: 33980746 Free PMC article. Review.
-
Adipose-Derived Stem Cell Exosomes Alleviate Psoriasis Serum Exosomes-Induced Inflammation by Regulating Autophagy and Redox Status in Keratinocytes.Clin Cosmet Investig Dermatol. 2023 Dec 23;16:3699-3711. doi: 10.2147/CCID.S439760. eCollection 2023. Clin Cosmet Investig Dermatol. 2023. PMID: 38152151 Free PMC article.
-
Potential Role of Cytochrome c and Tryptase in Psoriasis and Psoriatic Arthritis Pathogenesis: Focus on Resistance to Apoptosis and Oxidative Stress.Front Immunol. 2018 Oct 30;9:2363. doi: 10.3389/fimmu.2018.02363. eCollection 2018. Front Immunol. 2018. PMID: 30429845 Free PMC article. Review.
-
Psoriasis: Past, Present, and Future.J Invest Dermatol. 2019 Nov;139(11):e133-e142. doi: 10.1016/j.jid.2019.08.437. J Invest Dermatol. 2019. PMID: 31648690 Free PMC article. Review. No abstract available.
-
Mini Review: New Treatments in Psoriatic Arthritis. Focus on the IL-23/17 Axis.Front Pharmacol. 2019 Aug 6;10:872. doi: 10.3389/fphar.2019.00872. eCollection 2019. Front Pharmacol. 2019. PMID: 31447673 Free PMC article. Review.
References
-
- Austin L.M., Ozawa M., Kikuchi T., Walters I.B., and Krueger J.G.. 1999. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-γ, interleukin-2, and tumor necrosis factor-α, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J. Invest. Dermatol. 113:752–759. 10.1046/j.1523-1747.1999.00749.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

