Adipose-Derived Mesenchymal Stem Cells Reduce Lymphocytic Infiltration in a Rabbit Model of Induced Autoimmune Dacryoadenitis
- PMID: 27699412
- PMCID: PMC6016434
- DOI: 10.1167/iovs.15-17824
Adipose-Derived Mesenchymal Stem Cells Reduce Lymphocytic Infiltration in a Rabbit Model of Induced Autoimmune Dacryoadenitis
Abstract
Purpose: To investigate the immunoregulatory roles of adipose-derived mesenchymal stem cells (ADSCs) in autoimmune dacryoadenitis.
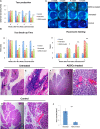
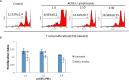
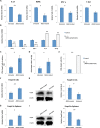
Methods: Rabbits were treated with ADSCs or phosphate-buffered solution on days 1, 3, 5, 7, and 9 after injection of activated peripheral blood lymphocytes, and clinical scores were determined by assessing tear production, break-up time, and fluorescein and hematoxylin and eosin staining. Inflammatory response was determined by measuring the expression of different mediators of inflammation in the lacrimal glands. The Th1/Th17-mediated autoreactive responses were evaluated by determining the proliferative response and the expression of cytokine genes and the lineage-determining transcription factors. The frequency of regulatory T cells (Tregs) was also examined.
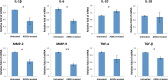
Results: The ADSC-treated rabbits showed decreased autoimmune responses, and the secretory function of their lacrimal gland was restored significantly. Treatment with ADSCs downregulated the Th1 and Th17 responses but enhanced Tregs function. In addition, ADSC treatment noticeably suppressed the expression of matrix metalloproteinase (MMP)-9, MPP-2, IL-1β, and IL-6, whereas it enhanced the expression of the anti-inflammatory cytokine IL-10.
Conclusions: Our results demonstrated that ADSC administration efficiently ameliorates autoimmune dacryoadenitis mainly via modulating Th1/Th17 responses.
Figures

Comment in
-
Adipose-Derived Mesenchymal Stem Cells Reduce Lymphocytic Infiltration in a Rabbit Model of Induced Autoimmune Dacryoadenitis: Some Discussions.Invest Ophthalmol Vis Sci. 2017 Mar 1;58(3):1585. doi: 10.1167/iovs.17-21694. Invest Ophthalmol Vis Sci. 2017. PMID: 28288268 No abstract available.
Similar articles
-
Tumor necrosis factor inhibitor gene expression suppresses lacrimal gland immunopathology in a rabbit model of autoimmune dacryoadenitis.Cornea. 2003 May;22(4):343-51. doi: 10.1097/00003226-200305000-00012. Cornea. 2003. PMID: 12792478
-
Lacrimal histopathology and ocular surface disease in a rabbit model of autoimmune dacryoadenitis.Cornea. 2003 Jan;22(1):25-32. doi: 10.1097/00003226-200301000-00007. Cornea. 2003. PMID: 12502944
-
Adeno-associated virus-mediated IL-10 gene transfer suppresses lacrimal gland immunopathology in a rabbit model of autoimmune dacryoadenitis.Invest Ophthalmol Vis Sci. 2010 Oct;51(10):5137-44. doi: 10.1167/iovs.10-5423. Epub 2010 May 26. Invest Ophthalmol Vis Sci. 2010. PMID: 20505195 Free PMC article.
-
Mesenchymal stem cells for treating autoimmune dacryoadenitis.Stem Cell Res Ther. 2017 Jun 5;8(1):126. doi: 10.1186/s13287-017-0593-3. Stem Cell Res Ther. 2017. PMID: 28583168 Free PMC article. Review.
-
Rabbit models of dry eye disease: Current understanding and unmet needs for translational research.Exp Eye Res. 2021 May;206:108538. doi: 10.1016/j.exer.2021.108538. Epub 2021 Mar 23. Exp Eye Res. 2021. PMID: 33771517 Review.
Cited by
-
Adipose Stem Cells in Modern-Day Ophthalmology.Clin Pract. 2023 Feb 4;13(1):230-245. doi: 10.3390/clinpract13010021. Clin Pract. 2023. PMID: 36826163 Free PMC article. Review.
-
MicroRNA expression profile of Lacrimal Glands in rabbit autoimmune dacryoadenitis model.Int J Med Sci. 2020 Oct 16;17(17):2879-2887. doi: 10.7150/ijms.50248. eCollection 2020. Int J Med Sci. 2020. PMID: 33162816 Free PMC article.
-
Exosomes From Human Umbilical Cord Mesenchymal Stem Cells Treat Corneal Injury via Autophagy Activation.Front Bioeng Biotechnol. 2022 Apr 11;10:879192. doi: 10.3389/fbioe.2022.879192. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35519619 Free PMC article.
-
Human umbilical cord mesenchymal stem cells alleviate ongoing autoimmune dacryoadenitis in rabbits via polarizing macrophages into an anti-inflammatory phenotype.Exp Eye Res. 2020 Feb;191:107905. doi: 10.1016/j.exer.2019.107905. Epub 2019 Dec 28. Exp Eye Res. 2020. PMID: 31891674 Free PMC article.
-
ADSCs stimulated by VEGF-C alleviate intestinal inflammation via dual mechanisms of enhancing lymphatic drainage by a VEGF-C/VEGFR-3-dependent mechanism and inhibiting the NF-κB pathway by the secretome.Stem Cell Res Ther. 2022 Sep 5;13(1):448. doi: 10.1186/s13287-022-03132-3. Stem Cell Res Ther. 2022. PMID: 36064450 Free PMC article.
References
-
- Fox RI. . Sjogren's syndrome. Lancet. 2005; 366: 321– 331. - PubMed
-
- Katsifis GE, Moutsopoulos NM, Wahl SM. . T lymphocytes in Sjogren's syndrome: contributors to and regulators of pathophysiology. Clin Rev Allergy Immunol. 2007; 32: 252– 264. - PubMed
-
- Li X, Li X, Qian L,et al. . T regulatory cells are markedly diminished in diseased salivary glands of patients with primary Sjogren's syndrome. J Rheumatol. 2007; 34: 2438– 2445. - PubMed
-
- Cornec D, Saraux A, Devauchelle-Pensec V, Clodic C, Pers JO. . The future of B cell-_targeted therapies in Sjogren's syndrome. Immunotherapy. 2013; 5: 639– 646. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

