Culture media profoundly affect Candida albicans and Candida tropicalis growth, adhesion and biofilm development
- PMID: 27706381
- PMCID: PMC5125054
- DOI: 10.1590/0074-02760160294
Culture media profoundly affect Candida albicans and Candida tropicalis growth, adhesion and biofilm development
Erratum in
-
ERRATUM.Mem Inst Oswaldo Cruz. 2020 Oct 19;115:e200294. doi: 10.1590/0074-02760200294ER. Mem Inst Oswaldo Cruz. 2020. PMID: 33084742 Free PMC article.
Abstract
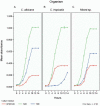
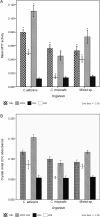
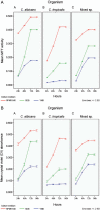
As there are sparse data on the impact of growth media on the phenomenon of biofilm development for Candida we evaluated the efficacy of three culture media on growth, adhesion and biofilm formation of two pathogenic yeasts, Candida albicans and Candida tropicalis. The planktonic phase yeast growth, either as monocultures or mixed cultures, in sabouraud dextrose broth (SDB), yeast nitrogen base (YNB), and RPMI 1640 was compared, and adhesion as well as biofilm formation were monitored using MTT and crystal violet (CV) assays and scanning electron microscopy. Planktonic cells of C. albicans, C. tropicalis and their 1:1 co-culture showed maximal growth in SDB. C. albicans/C. tropicalis adhesion was significantly facilitated in RPMI 1640 although the YNB elicited the maximum growth for C. tropicalis. Similarly, the biofilm growth was uniformly higher for both species in RPMI 1640, and C. tropicalis was the slower biofilm former in all three media. Scanning electron microscopy images tended to confirm the results of MTT and CV assay. Taken together, our data indicate that researchers should pay heed to the choice of laboratory culture media when comparing relative planktonic/biofilm growth of Candida. There is also a need for standardisation of biofilm development media so as to facilitate cross comparisons between laboratories.
Figures

Similar articles
-
Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis.J Mycol Med. 2020 Apr;30(1):100911. doi: 10.1016/j.mycmed.2019.100911. Epub 2019 Nov 7. J Mycol Med. 2020. PMID: 32008964
-
Evaluation of combined growth media for in vitro cultivation of oropharyngeal biofilms on prosthetic silicone.J Mater Sci Mater Med. 2018 Apr 9;29(4):45. doi: 10.1007/s10856-018-6051-7. J Mater Sci Mater Med. 2018. PMID: 29633010 Free PMC article.
-
The Effect of Nutritive and Non-Nutritive Sweeteners on the Growth, Adhesion, and Biofilm Formation of Candida albicans and Candida tropicalis.Med Princ Pract. 2017;26(6):554-560. doi: 10.1159/000484718. Epub 2017 Nov 1. Med Princ Pract. 2017. PMID: 29131083 Free PMC article.
-
Detailed comparison of Candida albicans and Candida glabrata biofilms under different conditions and their susceptibility to caspofungin and anidulafungin.J Med Microbiol. 2011 Sep;60(Pt 9):1261-1269. doi: 10.1099/jmm.0.032037-0. Epub 2011 May 12. J Med Microbiol. 2011. PMID: 21566087
-
Adhesion and biofilm formation by the opportunistic pathogen Candida tropicalis: what do we know?Can J Microbiol. 2023 Jun 1;69(6):207-218. doi: 10.1139/cjm-2022-0195. Epub 2023 Feb 21. Can J Microbiol. 2023. PMID: 36809069 Review.
Cited by
-
Curcumin as a Promising Antibacterial Agent: Effects on Metabolism and Biofilm Formation in S. mutans.Biomed Res Int. 2018 Feb 28;2018:4508709. doi: 10.1155/2018/4508709. eCollection 2018. Biomed Res Int. 2018. PMID: 29682545 Free PMC article.
-
Anticandida and antibiofilm activities of extract from Schinopsis brasiliensis Engl. against Candida spp.Braz Oral Res. 2024 Mar 11;38:e016. doi: 10.1590/1807-3107bor-2024.vol38.0016. eCollection 2024. Braz Oral Res. 2024. PMID: 38477802 Free PMC article.
-
Optotracing for live selective fluorescence-based detection of Candida albicans biofilms.Front Cell Infect Microbiol. 2022 Sep 2;12:981454. doi: 10.3389/fcimb.2022.981454. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36118028 Free PMC article.
-
Polymeric micelles with anti-virulence activity against Candida albicans in a single- and dual-species biofilm.Drug Deliv Transl Res. 2021 Aug;11(4):1586-1597. doi: 10.1007/s13346-021-00943-4. Epub 2021 Mar 13. Drug Deliv Transl Res. 2021. PMID: 33713317
-
Rosemary essential oil and its components 1,8-cineole and α-pinene induce ROS-dependent lethality and ROS-independent virulence inhibition in Candida albicans.PLoS One. 2022 Nov 16;17(11):e0277097. doi: 10.1371/journal.pone.0277097. eCollection 2022. PLoS One. 2022. PMID: 36383525 Free PMC article.
References
-
- Al-Fattani MA, Douglas LJ. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. 8J Med Microbiol. 2006;55:999–1008. - PubMed
-
- Bandara HM, Lam OL, Watt RM, Jin LJ, Samaranayake LP. Bacterial lipopolysaccharides variably modulate in vitro biofilm formation of Candida species. 10J Med Microbiol. 2010;59:1225–1234. - PubMed
-
- Bizerra FC, Nakamura CV, Poersch C, Svidzinski TIE, Quesada RMB, Goldenberg S, et al. Characteristics of biofilm formation by Candida tropicalis and antifungal resistance. FEMS Yeast Res. 2008;8(3):442–450. - PubMed
-
- Cannon RD, Holmes AR, Mason AB, Monk BC. Oral Candida: clearance, colonization, or candidiasis? J Dent Res. 1995;74(5):1152–1161. - PubMed
-
- CLSI - Clinical and Laboratory Standards Institiute . CLSI document M27-A3. Third Edition. Wayne: CLSI; 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved Standard.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

