The struggle by Caenorhabditis elegans to maintain proteostasis during aging and disease
- PMID: 27809888
- PMCID: PMC5093949
- DOI: 10.1186/s13062-016-0161-2
The struggle by Caenorhabditis elegans to maintain proteostasis during aging and disease
Abstract
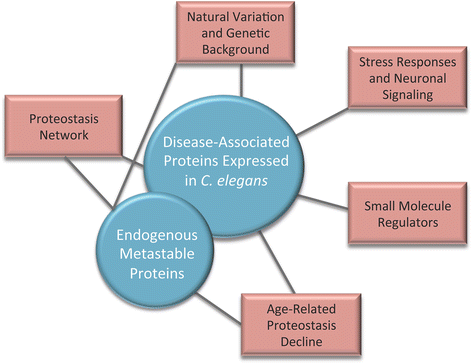
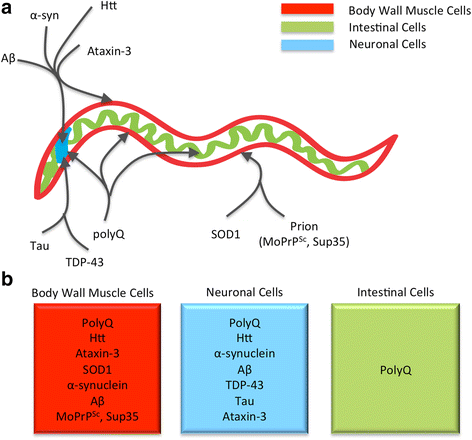
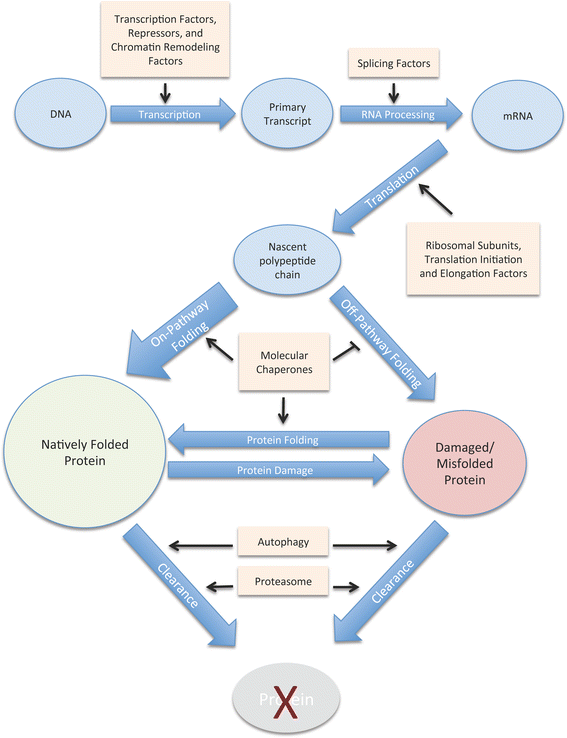
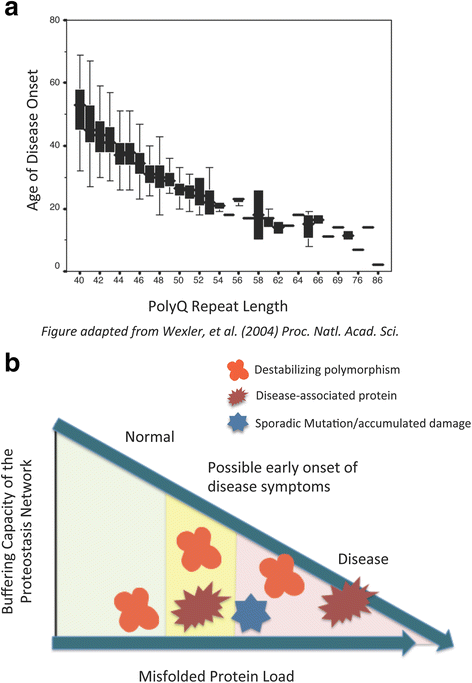
The presence of only small amounts of misfolded protein is an indication of a healthy proteome. Maintaining proteome health, or more specifically, "proteostasis," is the purview of the "proteostasis network." This network must respond to constant fluctuations in the amount of destabilized proteins caused by errors in protein synthesis and exposure to acute proteotoxic conditions. Aging is associated with a gradual increase in damaged and misfolded protein, which places additional stress on the machinery of the proteostasis network. In fact, despite the ability of the proteostasis machinery to readjust its stoichiometry in an attempt to maintain homeostasis, the capacity of cells to buffer against misfolding is strikingly limited. Therefore, subtle changes in the folding environment that occur during aging can significantly impact the health of the proteome. This decline and eventual collapse in proteostasis is most pronounced in individuals with neurodegenerative disorders such as Alzheimer's Disease, Parkinson's Disease, and Huntington's Disease that are caused by the misfolding, aggregation, and toxicity of certain proteins. This review discusses how C. elegans models of protein misfolding have contributed to our current understanding of the proteostasis network, its buffering capacity, and its regulation.
Reviewers: This article was reviewed by Luigi Bubacco, Patrick Lewis and Xavier Roucou.
Figures
Similar articles
-
Homeodomain-interacting protein kinase maintains neuronal homeostasis during normal Caenorhabditis elegans aging and systemically regulates longevity from serotonergic and GABAergic neurons.Elife. 2023 Jun 20;12:e85792. doi: 10.7554/eLife.85792. Elife. 2023. PMID: 37338980 Free PMC article.
-
Organismal Protein Homeostasis Mechanisms.Genetics. 2020 Aug;215(4):889-901. doi: 10.1534/genetics.120.301283. Genetics. 2020. PMID: 32759342 Free PMC article. Review.
-
Determining the effects of nanoparticulate air pollution on proteostasis in Caenorhabditis elegans.PLoS One. 2020 Dec 3;15(12):e0243419. doi: 10.1371/journal.pone.0243419. eCollection 2020. PLoS One. 2020. Update in: PLoS One. 2023 Feb 23;18(2):e0275137. doi: 10.1371/journal.pone.0275137 PMID: 33270781 Free PMC article. Updated.
-
The intrinsic and extrinsic factors that contribute to proteostasis decline and pathological protein misfolding.Adv Protein Chem Struct Biol. 2019;118:145-161. doi: 10.1016/bs.apcsb.2019.07.001. Epub 2019 Nov 26. Adv Protein Chem Struct Biol. 2019. PMID: 31928724 Review.
-
Endoplasmic reticulum proteostasis impairment in aging.Aging Cell. 2017 Aug;16(4):615-623. doi: 10.1111/acel.12599. Epub 2017 Apr 23. Aging Cell. 2017. PMID: 28436203 Free PMC article. Review.
Cited by
-
Nature Versus Nurture: Does Proteostasis Imbalance Underlie the Genetic, Environmental, and Age-Related Risk Factors for Alzheimer's Disease?Healthcare (Basel). 2017 Aug 22;5(3):46. doi: 10.3390/healthcare5030046. Healthcare (Basel). 2017. PMID: 28829364 Free PMC article. Review.
-
Modeling Alzheimer's Disease in Caenorhabditis elegans.Biomedicines. 2022 Jan 26;10(2):288. doi: 10.3390/biomedicines10020288. Biomedicines. 2022. PMID: 35203497 Free PMC article. Review.
-
A unifying hypothesis on the central role of reactive oxygen species in bacterial pathogenesis and host defense in C. elegans.Curr Opin Immunol. 2021 Feb;68:9-20. doi: 10.1016/j.coi.2020.08.002. Epub 2020 Sep 6. Curr Opin Immunol. 2021. PMID: 32898751 Free PMC article. Review.
-
A new Caenorhabditis elegans model of human huntingtin 513 aggregation and toxicity in body wall muscles.PLoS One. 2017 Mar 10;12(3):e0173644. doi: 10.1371/journal.pone.0173644. eCollection 2017. PLoS One. 2017. PMID: 28282438 Free PMC article.
-
Characterization of Amyloid Structures in Aging C. Elegans Using Fluorescence Lifetime Imaging.J Vis Exp. 2020 Mar 27;(157):10.3791/61004. doi: 10.3791/61004. J Vis Exp. 2020. PMID: 32281971 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

