Synthesis and Cytotoxic Evaluation of Pyrrole Hetarylazoles Containing Benzimidazole/Pyrazolone/1,3,4-Oxadiazole Motifs
- PMID: 27956751
- PMCID: PMC5147751
- DOI: 10.1002/jhet.2501
Synthesis and Cytotoxic Evaluation of Pyrrole Hetarylazoles Containing Benzimidazole/Pyrazolone/1,3,4-Oxadiazole Motifs
Abstract
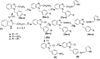
Azomethine linked pyrrole bishetarylazoles containing benzimidazole/pyrazolone/1,3,4-oxadiazole were synthesized in satisfactory yields. Their structures were confirmed by IR, 1H-NMR, 13C-NMR and elemental analysis. Evaluation for the cytotoxic activities In vitro against a panel of breast cancer cell lines (MDA-AB-231, BT-474 and Ishikawa cells) revealed that the pyrrole-benzimidazole hybrids are more potent than the pyrazolone and 1,3,4-oxadiazole hybrids in all cell lines. Compound (9) displayed promising cytotoxicity against BT-474 cell line with IC50 values, 7.7 µM.
Figures
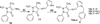
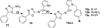
Similar articles
-
Thieno[2,3-d]pyrimidin-4(3H)-one Derivatives of Benzimidazole as Potential Anti- Breast Cancer (MDA-MB-231, MCF-7) Agents.Anticancer Agents Med Chem. 2021;21(11):1441-1450. doi: 10.2174/1871520620666200721131431. Anticancer Agents Med Chem. 2021. PMID: 32698751
-
Synthesis, anticancer evaluation and molecular docking studies of new benzimidazole- 1,3,4-oxadiazole derivatives as human topoisomerase types I poison.J Enzyme Inhib Med Chem. 2020 Dec;35(1):1657-1673. doi: 10.1080/14756366.2020.1806831. J Enzyme Inhib Med Chem. 2020. PMID: 32811204 Free PMC article.
-
New 1,3,4-oxadiazole-chalcone/benzimidazole hybrids as potent antiproliferative agents.Arch Pharm (Weinheim). 2023 Feb;356(2):e2200357. doi: 10.1002/ardp.202200357. Epub 2022 Nov 9. Arch Pharm (Weinheim). 2023. PMID: 36351754
-
New Pyrrole Derivatives as Promising Biological Agents: Design, Synthesis, Characterization, In Silico, and Cytotoxicity Evaluation.Int J Mol Sci. 2022 Aug 9;23(16):8854. doi: 10.3390/ijms23168854. Int J Mol Sci. 2022. PMID: 36012121 Free PMC article.
-
Cinnamide derived pyrimidine-benzimidazole hybrids as tubulin inhibitors: Synthesis, in silico and cell growth inhibition studies.Bioorg Chem. 2021 May;110:104765. doi: 10.1016/j.bioorg.2021.104765. Epub 2021 Feb 24. Bioorg Chem. 2021. PMID: 33677248
Cited by
-
Hybrids of Benzimidazole-oxadiazole: A New Avenue for Synthesis, Pharmacological Activity and Recent Patents for the Development of More Effective Ligands.Curr Org Synth. 2024;21(8):976-1013. doi: 10.2174/0115701794260740231010111408. Curr Org Synth. 2024. PMID: 37916627 Review.
-
Improving Representation of Underrepresented Minority (URM) Students in Oncology Biomedical Research Workforce: Outcome Evaluation from the ReTOOL Program.J Cancer Educ. 2022 Feb;37(1):37-45. doi: 10.1007/s13187-020-01779-1. J Cancer Educ. 2022. PMID: 32533539 Free PMC article.
References
-
- Kumar V, Kaue K, Gupta GK, Sharma AK. Eur. J. Med. Chem. 2013;69:735–753. - PubMed
-
- Spasov A, L Yozhitsa I, Bugaeva LI, Amisimova VA. Pharm. Chem. J. 1999;33(5):232.
-
- Gaba Monika, Singh Sarbjot, Mohan Chander. Eur. J. Med. Chem. 2014;76(9):494. - PubMed
-
- Khokra SI, Choudhary D. Asian J. Biochem. Pharm. Res. 2011;3(1):476.
-
- Kharb R, ShaharYar M, Sharma PC. Mini Rev Med Chem. 2011;11(1):84. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
