Relationship of glycated haemoglobin and reported hypoglycaemia to cardiovascular outcomes in patients with type 2 diabetes and recent acute coronary syndrome events: The EXAMINE trial
- PMID: 28058763
- PMCID: PMC5836868
- DOI: 10.1111/dom.12871
Relationship of glycated haemoglobin and reported hypoglycaemia to cardiovascular outcomes in patients with type 2 diabetes and recent acute coronary syndrome events: The EXAMINE trial
Erratum in
-
Corrigendum.Diabetes Obes Metab. 2017 Sep;19(9):1333. doi: 10.1111/dom.13076. Diabetes Obes Metab. 2017. PMID: 28834341 Free PMC article. No abstract available.
Abstract
Aims: To investigate relationships between glycated haemoglobin (HbA1c) and reported hypoglycaemia and risk of major adverse cardiovascular events (MACE).
Methods: The EXAMINE trial randomized 5380 patients with type 2 diabetes (T2DM) and a recent acute coronary syndrome (ACS) event, in 49 countries, to double-blind treatment with alogliptin or placebo in addition to standard of care. We used Cox proportional hazards models to analyse relationships among MACE, HbA1c levels and hypoglycaemic events.
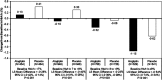
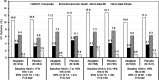
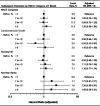
Results: Patients randomized to alogliptin achieved lower HbA1c levels than the placebo group in all baseline HbA1c categories without differences in hypoglycaemia rates. No systematic change was found in MACE rates according to baseline HbA1c (Pinteraction = 0.971) or HbA1c category at 1 month. Patients in the combined treatment groups (n = 5380) who experienced serious hypoglycaemia (n = 34) had higher MACE rates than those who did not (35.3% vs 11.4%, adjusted hazard ratio [HR] 2.42, 95% confidence interval [CI] 1.27-4.60; P = .007), although the association was less strong when analysing only events after the hypoglycaemic event (adjusted HR 1.60, 95% CI 0.80, 3.20).
Conclusions: There were no relationships between baseline HbA1c levels or HbA1c levels after 1 month of treatment and the risk of MACE. Alogliptin improved glycaemic control without increasing hypoglycaemia. Reported events of hypoglycaemia and serious hypoglycaemia were associated with MACE. These data underscore the safety of alogliptin in improving glycaemic control in T2DM post-ACS. Further study of hypoglycaemia as an independent risk factor for MACE in patients with T2DM and coronary disease is needed.
Keywords: HbA1c; alogliptin; cardiovascular disease; coronary disease; diabetes; hypoglycaemia; myocardial infarction; stroke.
© 2017 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
S.R. Heller: Research Grant; Takeda, NovoNordisk, Eli Lilly, Sanofi Aventis. Consultant/Advisory Board; Takeda, NovoNordisk, Eli Lilly. R.M. Bergenstal: Consultant/Advisory Board; Abbott Diabetes Care, Amylin, AstraZeneca, Bayer, Takeda, Becton Dickinson, Calibra, Eli Lilly, Halozyme, Johnson and Johnson, Medronic, NovoNordisk, Resmed, Roche, Sanofi. W.B. White: consulting/personal fees as Chair, Steering Committee for EXAMINE, Takeda. S. Kupfer: Full time employee of Takeda. G.L. Bakris: Research Grant; Takeda, Medtronic, Relypsa. Consultant/Advisory Board; Abbvie, CVRx, Janssen, Eli Lilly, Medtronic, Novartis, GSK, Bayer. W.C. Cushman: Research Grant; Boehringer‐Ingelheim, Lilly. C.R. Mehta: None. S.E. Nissen: None. C. Wilson: Full time employee of Takeda. F. Zannad: Consultant/Advisory Board; Servier, Resmed, Janssen, Novartis, Air Liquide, Cardiorenal diagnostics, CVCT, Biotronik, St. Jude, Boston Scientific. Consultant/Advisory Board; Takeda, Pfizer. Yuyin Liu: Full time employee of Takeda. Noah M. Gourlie: Full time employee of Takeda. C.P. Cannon: Research Grant; Takeda, Accumetrics, Arisaph, Astra Zeneca, Boehringer‐Ingelheim, GlaxoSmithKline, Janssen, Merck, Regeneron, Sanofi. Consultant/Advisory Board; Modest; CSL Behring, Essentials, Takeda.
Figures

Similar articles
-
Relationship of glycaemic control and hypoglycaemic episodes to 4-year cardiovascular outcomes in people with type 2 diabetes starting insulin.Diabetes Obes Metab. 2016 Feb;18(2):152-8. doi: 10.1111/dom.12598. Epub 2015 Dec 23. Diabetes Obes Metab. 2016. PMID: 26511332 Free PMC article.
-
Cardiovascular safety of the dipetidyl peptidase-4 inhibitor alogliptin in type 2 diabetes mellitus.Diabetes Obes Metab. 2013 Jul;15(7):668-73. doi: 10.1111/dom.12093. Epub 2013 Apr 4. Diabetes Obes Metab. 2013. PMID: 23489301 Clinical Trial.
-
Efficacy and safety of fixed-dose combination therapy, alogliptin plus metformin, in Asian patients with type 2 diabetes: A phase 3 trial.Diabetes Obes Metab. 2017 May;19(5):754-758. doi: 10.1111/dom.12875. Epub 2017 Feb 22. Diabetes Obes Metab. 2017. PMID: 28075066 Free PMC article. Clinical Trial.
-
Comparative review of dipeptidyl peptidase-4 inhibitors and sulphonylureas.Diabetes Obes Metab. 2016 Apr;18(4):333-47. doi: 10.1111/dom.12610. Epub 2016 Jan 8. Diabetes Obes Metab. 2016. PMID: 26597596 Review.
-
Prevention of cardiovascular disease through reduction of glycaemic exposure in type 2 diabetes: A perspective on glucose-lowering interventions.Diabetes Obes Metab. 2018 Feb;20(2):238-244. doi: 10.1111/dom.13033. Epub 2017 Jul 25. Diabetes Obes Metab. 2018. PMID: 28597588 Review.
Cited by
-
Association between Hemoglobin A1c and Stroke Risk in Patients with Type 2 Diabetes.J Stroke. 2020 Jan;22(1):87-98. doi: 10.5853/jos.2019.01704. Epub 2020 Jan 31. J Stroke. 2020. PMID: 32027794 Free PMC article.
-
Benefit-Risk Assessment of Alogliptin for the Treatment of Type 2 Diabetes Mellitus.Drug Saf. 2019 Nov;42(11):1311-1327. doi: 10.1007/s40264-019-00857-8. Drug Saf. 2019. PMID: 31654243 Free PMC article. Review.
-
Dipeptidyl peptidase-4 inhibitors, glucagon-like peptide 1 receptor agonists and sodium-glucose co-transporter-2 inhibitors for people with cardiovascular disease: a network meta-analysis.Cochrane Database Syst Rev. 2021 Oct 25;10(10):CD013650. doi: 10.1002/14651858.CD013650.pub2. Cochrane Database Syst Rev. 2021. PMID: 34693515 Free PMC article.
-
Day-to-day fasting glycaemic variability in DEVOTE: associations with severe hypoglycaemia and cardiovascular outcomes (DEVOTE 2).Diabetologia. 2018 Jan;61(1):48-57. doi: 10.1007/s00125-017-4423-z. Epub 2017 Sep 15. Diabetologia. 2018. PMID: 28913575 Free PMC article. Clinical Trial.
-
Relationship between hypoglycaemia, cardiovascular outcomes, and empagliflozin treatment in the EMPA-REG OUTCOME® trial.Eur Heart J. 2020 Jan 7;41(2):209-217. doi: 10.1093/eurheartj/ehz621. Eur Heart J. 2020. PMID: 31504427 Free PMC article. Clinical Trial.
References
-
- Wright RJ, Frier BM. Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes Metab Res Rev. 2008;24:353–363. - PubMed
-
- Chow E, Bernjak A, Williams S, et al. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes. 2014;63:1738–1747. - PubMed
-
- Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta‐analysis with bias analysis. BMJ. 2013;347:f4533. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

