Racial Variation in the Utility of Urinary Biomarkers PCA3 and T2ERG in a Large Multicenter Study
- PMID: 28115190
- PMCID: PMC5568076
- DOI: 10.1016/j.juro.2017.01.058
Racial Variation in the Utility of Urinary Biomarkers PCA3 and T2ERG in a Large Multicenter Study
Abstract
Purpose: To our knowledge it is unknown whether urinary biomarkers for prostate cancer have added utility to clinical risk calculators in different racial groups. We examined the utility of urinary biomarkers added to clinical risk calculators for predicting prostate cancer in African American and nonAfrican American men.
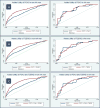
Materials and methods: Demographics, PCPT (Prostate Cancer Prevention Trial) risk scores, data on the biomarkers data PCA3 (prostate cancer antigen 3) and T2ERG (transmembrane protease serine 2 and v-ets erythroblastosis virus E26 oncogene homolog gene fusion), and biopsy pathology features were prospectively collected on 718 men as part of EDRN (Early Detection Research Network). Utility was determined by generating ROC curves and comparing AUC values for the baseline multivariable PCPT model and for models containing biomarker scores.
Results: PCA3 and T2ERG added utility for the prediction of prostate cancer and clinically significant prostate cancer when combined with the PCPT Risk Calculator. This utility was seen in nonAfrican American men only for PCA3 (AUC 0.64 increased to 0.75 for prostate cancer and to 0.69-0.77 for clinically significant prostate cancer, both p <0.001) and for T2ERG (AUC 0.64-0.74 for prostate cancer, p <0.001, and 0.69-0.73 for clinically significant prostate cancer, p = 0.029). African American men did not have an added benefit with the addition of biomarkers, including PCA3 (AUC 0.75-0.77, p = 0.64, and 0.65-0.66, p = 0.74) and T2ERG (AUC 0.75-0.74, p = 0.74, and 0.65-0.64, p = 0.88), for prostate cancer and clinically significant prostate cancer, respectively. Limitations include the small number of African American men (72). The post hoc subgroup analysis nature of the study limited findings to being hypothesis generating.
Conclusions: As novel biomarkers are discovered, clinical utility should be established across demographically diverse cohorts.
Keywords: African Americans; biomarkers; gene fusion; human; prostate cancer antigen 3; prostatic neoplasms; tumor.
Copyright © 2017 American Urological Association Education and Research, Inc. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Editorial Comment.J Urol. 2017 Jul;198(1):48-49. doi: 10.1016/j.juro.2017.01.094. Epub 2017 Mar 23. J Urol. 2017. PMID: 28344032 No abstract available.
Similar articles
-
Clinical Use of PCA3 and TMPRSS2:ERG Urinary Biomarkers in African-American Men Undergoing Prostate Biopsy.J Urol. 2016 Oct;196(4):1053-60. doi: 10.1016/j.juro.2016.04.075. Epub 2016 Apr 29. J Urol. 2016. PMID: 27140073
-
Prospective multicentre evaluation of PCA3 and TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer.Eur Urol. 2014 Mar;65(3):534-42. doi: 10.1016/j.eururo.2012.11.014. Epub 2012 Nov 15. Eur Urol. 2014. PMID: 23201468
-
Incorporation of Urinary Prostate Cancer Antigen 3 and TMPRSS2:ERG into Prostate Cancer Prevention Trial Risk Calculator.Eur Urol Focus. 2019 Jan;5(1):54-61. doi: 10.1016/j.euf.2018.01.010. Epub 2018 Feb 13. Eur Urol Focus. 2019. PMID: 29422418 Free PMC article.
-
Urinary Prostate Cancer Antigen 3 as a Tumour Marker: Biochemical and Clinical Aspects.Adv Exp Med Biol. 2015;867:277-89. doi: 10.1007/978-94-017-7215-0_17. Adv Exp Med Biol. 2015. PMID: 26530372 Review.
-
Blood-based and urinary prostate cancer biomarkers: a review and comparison of novel biomarkers for detection and treatment decisions.Prostate Cancer Prostatic Dis. 2017 Mar;20(1):12-19. doi: 10.1038/pcan.2016.59. Epub 2016 Dec 6. Prostate Cancer Prostatic Dis. 2017. PMID: 27922627 Review.
Cited by
-
Identification of a novel non-invasive biological marker to overcome the shortcomings of PSA in diagnosis and risk stratification for prostate cancer: Initial prospective study of developmental endothelial locus-1 protein.PLoS One. 2021 Apr 26;16(4):e0250254. doi: 10.1371/journal.pone.0250254. eCollection 2021. PLoS One. 2021. PMID: 33901217 Free PMC article.
-
Prostate cancer knowledge and barriers to screening among men at risk in northern Tanzania: A community-based study.Cancer Treat Res Commun. 2024;39:100811. doi: 10.1016/j.ctarc.2024.100811. Epub 2024 Apr 2. Cancer Treat Res Commun. 2024. PMID: 38574439 Free PMC article.
-
Molecular Biomarkers for the Detection of Clinically Significant Prostate Cancer: A Systematic Review and Meta-analysis.Eur Urol Open Sci. 2022 Nov 10;46:105-127. doi: 10.1016/j.euros.2022.10.017. eCollection 2022 Dec. Eur Urol Open Sci. 2022. PMID: 36388432 Free PMC article. Review.
-
Biomarkers for prostate cancer detection and risk stratification.Ther Adv Urol. 2022 Jun 14;14:17562872221103988. doi: 10.1177/17562872221103988. eCollection 2022 Jan-Dec. Ther Adv Urol. 2022. PMID: 35719272 Free PMC article. Review.
-
Trend in incidence and clinicopathological characteristics of prostate cancer in Northern Tanzania: analysis from a population based cancer registry data 2015-2021.BMC Cancer. 2024 Nov 19;24(1):1424. doi: 10.1186/s12885-024-13194-6. BMC Cancer. 2024. PMID: 39563287 Free PMC article.
References
-
- Siegel R, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. - PubMed
-
- Louie KS, et al. Do prostate cancer risk models improve the predictive accuracy of PSA screening? A meta-analysis. Ann Oncol. 2014 - PubMed
-
- Fedewa SA, et al. Association of insurance and race/ethnicity with disease severity among men diagnosed with prostate cancer, National Cancer Database 2004–2006. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2437–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

