Mutations in EXTL3 Cause Neuro-immuno-skeletal Dysplasia Syndrome
- PMID: 28132690
- PMCID: PMC5294674
- DOI: 10.1016/j.ajhg.2017.01.013
Mutations in EXTL3 Cause Neuro-immuno-skeletal Dysplasia Syndrome
Abstract
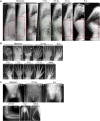
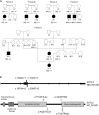
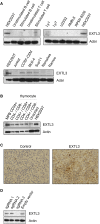
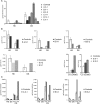
EXTL3 regulates the biosynthesis of heparan sulfate (HS), important for both skeletal development and hematopoiesis, through the formation of HS proteoglycans (HSPGs). By whole-exome sequencing, we identified homozygous missense mutations c.1382C>T, c.1537C>T, c.1970A>G, and c.2008T>G in EXTL3 in nine affected individuals from five unrelated families. Notably, we found the identical homozygous missense mutation c.1382C>T (p.Pro461Leu) in four affected individuals from two unrelated families. Affected individuals presented with variable skeletal abnormalities and neurodevelopmental defects. Severe combined immunodeficiency (SCID) with a complete absence of T cells was observed in three families. EXTL3 was most abundant in hematopoietic stem cells and early progenitor T cells, which is in line with a SCID phenotype at the level of early T cell development in the thymus. To provide further support for the hypothesis that mutations in EXTL3 cause a neuro-immuno-skeletal dysplasia syndrome, and to gain insight into the pathogenesis of the disorder, we analyzed the localization of EXTL3 in fibroblasts derived from affected individuals and determined glycosaminoglycan concentrations in these cells as well as in urine and blood. We observed abnormal glycosaminoglycan concentrations and increased concentrations of the non-sulfated chondroitin disaccharide D0a0 and the disaccharide D0a4 in serum and urine of all analyzed affected individuals. In summary, we show that biallelic mutations in EXTL3 disturb glycosaminoglycan synthesis and thus lead to a recognizable syndrome characterized by variable expression of skeletal, neurological, and immunological abnormalities.
Keywords: EXTL3; T cell SCID; exostosin; heparan sulfate; neuro-immuno-skeletal dysplasia.
Copyright © 2017 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Immune skeletal dysplasia with neurodevelopmental abnormalities caused by a novel variant of EXTL3 gene in a Chinese family.Mol Genet Genomic Med. 2024 Jan;12(1):e2308. doi: 10.1002/mgg3.2308. Epub 2023 Nov 27. Mol Genet Genomic Med. 2024. PMID: 38010033 Free PMC article.
-
EXTL3-Associated Immunoskeletal Dysplasia with Neurodevelopmental Abnormalities: A Lethal Phenotype.Pediatr Allergy Immunol Pulmonol. 2023 Dec;36(4):147-149. doi: 10.1089/ped.2023.0079. Epub 2023 Nov 20. Pediatr Allergy Immunol Pulmonol. 2023. PMID: 38010729
-
An ultra-rare case of immunoskeletal dysplasia with neurodevelopmental abnormalities in an Indian patient with homozygous c.953C > T variant in EXTL3 gene: a case report.BMC Pediatr. 2022 Feb 3;22(1):78. doi: 10.1186/s12887-022-03143-2. BMC Pediatr. 2022. PMID: 35114981 Free PMC article. Review.
-
Identification of biallelic EXTL3 mutations in a novel type of spondylo-epi-metaphyseal dysplasia.J Hum Genet. 2017 Aug;62(8):797-801. doi: 10.1038/jhg.2017.38. Epub 2017 Mar 23. J Hum Genet. 2017. PMID: 28331220 Free PMC article.
-
Specific functions of Exostosin-like 3 (EXTL3) gene products.Cell Mol Biol Lett. 2020 Aug 20;25:39. doi: 10.1186/s11658-020-00231-y. eCollection 2020. Cell Mol Biol Lett. 2020. PMID: 32843889 Free PMC article. Review.
Cited by
-
CSGALNACT1-congenital disorder of glycosylation: A mild skeletal dysplasia with advanced bone age.Hum Mutat. 2020 Mar;41(3):655-667. doi: 10.1002/humu.23952. Epub 2019 Dec 3. Hum Mutat. 2020. PMID: 31705726 Free PMC article.
-
Clinical, Immunological, and Molecular Features of Typical and Atypical Severe Combined Immunodeficiency: Report of the Italian Primary Immunodeficiency Network.Front Immunol. 2019 Aug 13;10:1908. doi: 10.3389/fimmu.2019.01908. eCollection 2019. Front Immunol. 2019. PMID: 31456805 Free PMC article.
-
Inherited Proteoglycan Biosynthesis Defects-Current Laboratory Tools and Bikunin as a Promising Blood Biomarker.Genes (Basel). 2021 Oct 20;12(11):1654. doi: 10.3390/genes12111654. Genes (Basel). 2021. PMID: 34828260 Free PMC article. Review.
-
The KOUNCIL Consortium: From Genetic Defects to Therapeutic Development for Nephronophthisis.Front Pediatr. 2018 May 7;6:131. doi: 10.3389/fped.2018.00131. eCollection 2018. Front Pediatr. 2018. PMID: 29868523 Free PMC article.
-
Advances and highlights in primary immunodeficiencies in 2017.J Allergy Clin Immunol. 2018 Oct;142(4):1041-1051. doi: 10.1016/j.jaci.2018.08.016. Epub 2018 Aug 29. J Allergy Clin Immunol. 2018. PMID: 30170128 Free PMC article. Review.
References
-
- Picard C., Al-Herz W., Bousfiha A., Casanova J.L., Chatila T., Conley M.E., Cunningham-Rundles C., Etzioni A., Holland S.M., Klein C. Primary Immunodeficiency Diseases: an Update on the Classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015. J. Clin. Immunol. 2015;35:696–726. - PMC - PubMed
-
- Ridanpää M., van Eenennaam H., Pelin K., Chadwick R., Johnson C., Yuan B., vanVenrooij W., Pruijn G., Salmela R., Rockas S. Mutations in the RNA component of RNase MRP cause a pleiotropic human disease, cartilage-hair hypoplasia. Cell. 2001;104:195–203. - PubMed
-
- Boerkoel C.F., Takashima H., John J., Yan J., Stankiewicz P., Rosenbarker L., André J.L., Bogdanovic R., Burguet A., Cockfield S. Mutant chromatin remodeling protein SMARCAL1 causes Schimke immuno-osseous dysplasia. Nat. Genet. 2002;30:215–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

