Perivascular extracellular matrix hydrogels mimic native matrix microarchitecture and promote angiogenesis via basic fibroblast growth factor
- PMID: 28167392
- PMCID: PMC5319845
- DOI: 10.1016/j.biomaterials.2017.01.037
Perivascular extracellular matrix hydrogels mimic native matrix microarchitecture and promote angiogenesis via basic fibroblast growth factor
Abstract
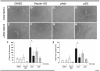
Extracellular matrix (ECM)-derived bioscaffolds have been shown to elicit tissue repair through retention of bioactive signals. Given that the adventitia of large blood vessels is a richly vascularized microenvironment, we hypothesized that perivascular ECM contains bioactive signals that influence cells of blood vessel lineages. ECM bioscaffolds were derived from decellularized human and porcine aortic adventitia (hAdv and pAdv, respectively) and then shown have minimal DNA content and retain elastin and collagen proteins. Hydrogel formulations of hAdv and pAdv ECM bioscaffolds exhibited gelation kinetics similar to ECM hydrogels derived from porcine small intestinal submucosa (pSIS). hAdv and pAdv ECM hydrogels displayed thinner, less undulated, and fibrous microarchitecture reminiscent of native adventitia, with slight differences in ultrastructure visible in comparison to pSIS ECM hydrogels. Pepsin-digested pAdv and pSIS ECM bioscaffolds increased proliferation of human adventitia-derived endothelial cells and this effect was mediated in part by basic fibroblast growth factor (FGF2). Human endothelial cells cultured on Matrigel substrates formed more numerous and longer tube-like structures when supplemented with pAdv ECM bioscaffolds, and FGF2 mediated this matrix signaling. ECM bioscaffolds derived from pAdv promoted FGF2-dependent in vivo angiogenesis in the chick chorioallantoic membrane model. Using an angiogenesis-focused protein array, we detected 55 angiogenesis-related proteins, including FGF2 in hAdv, pAdv and pSIS ECMs. Interestingly, 19 of these factors were less abundant in ECMs bioscaffolds derived from aneurysmal specimens of human aorta when compared with non-aneurysmal (normal) specimens. This study reveals that Adv ECM hydrogels recapitulate matrix fiber microarchitecture of native adventitia, and retain angiogenesis-related actors and bioactive properties such as FGF2 signaling capable of influencing processes important for angiogenesis. This work supports the use of Adv ECM bioscaffolds for both discovery biology and potential translation towards microvascular regeneration in clinical applications.
Keywords: Adventitia; Aneurysm; Angiogenesis; Endothelial cell; Extracellular matrix; Hydrogel; Tube formation.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures


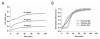
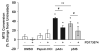


Similar articles
-
Native extracellular matrix/fibroin hydrogels for adipose tissue engineering with enhanced vascularization.Biomed Mater. 2017 Jun 6;12(3):035007. doi: 10.1088/1748-605X/aa6a63. Biomed Mater. 2017. PMID: 28361795
-
Hydrogel derived from porcine decellularized nerve tissue as a promising biomaterial for repairing peripheral nerve defects.Acta Biomater. 2018 Jun;73:326-338. doi: 10.1016/j.actbio.2018.04.001. Epub 2018 Apr 9. Acta Biomater. 2018. PMID: 29649641
-
Decellularized Annulus Fibrosus Matrix/Chitosan Hybrid Hydrogels with Basic Fibroblast Growth Factor for Annulus Fibrosus Tissue Engineering.Tissue Eng Part A. 2019 Dec;25(23-24):1605-1613. doi: 10.1089/ten.TEA.2018.0297. Epub 2019 Nov 21. Tissue Eng Part A. 2019. PMID: 30929614 Free PMC article.
-
Whole Cardiac Tissue Bioscaffolds.Adv Exp Med Biol. 2018;1098:85-114. doi: 10.1007/978-3-319-97421-7_5. Adv Exp Med Biol. 2018. PMID: 30238367 Review.
-
Extracellular matrix hydrogels from decellularized tissues: Structure and function.Acta Biomater. 2017 Feb;49:1-15. doi: 10.1016/j.actbio.2016.11.068. Epub 2016 Dec 1. Acta Biomater. 2017. PMID: 27915024 Free PMC article. Review.
Cited by
-
Using of Single-Layer Porcine Small Intestinal Submucosa in Urethroplasty on a Beagle Model.Biomed Res Int. 2022 Sep 27;2022:1755886. doi: 10.1155/2022/1755886. eCollection 2022. Biomed Res Int. 2022. PMID: 36203480 Free PMC article.
-
On vasa vasorum: A history of advances in understanding the vessels of vessels.Sci Adv. 2022 Apr 22;8(16):eabl6364. doi: 10.1126/sciadv.abl6364. Epub 2022 Apr 20. Sci Adv. 2022. PMID: 35442731 Free PMC article. Review.
-
Editorial: Exploring the Frontiers of Regenerative Cardiovascular Medicine.Front Cardiovasc Med. 2019 Feb 26;6:13. doi: 10.3389/fcvm.2019.00013. eCollection 2019. Front Cardiovasc Med. 2019. PMID: 30873414 Free PMC article. No abstract available.
-
Sterilization and disinfection methods for decellularized matrix materials: Review, consideration and proposal.Bioact Mater. 2021 Feb 27;6(9):2927-2945. doi: 10.1016/j.bioactmat.2021.02.010. eCollection 2021 Sep. Bioact Mater. 2021. PMID: 33732964 Free PMC article. Review.
-
A Natural Xenogeneic Endometrial Extracellular Matrix Hydrogel Toward Improving Current Human in vitro Models and Future in vivo Applications.Front Bioeng Biotechnol. 2021 Mar 5;9:639688. doi: 10.3389/fbioe.2021.639688. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33748086 Free PMC article.
References
-
- Londono R, Badylak SF. Biologic Scaffolds for Regenerative Medicine: Mechanisms of In vivo Remodeling. Ann Biomed Eng. 2015;43:577–92. - PubMed
-
- Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta biomaterialia. 2009;5:1–13. - PubMed
-
- Singelyn JM, Sundaramurthy P, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM, et al. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J Am Coll Cardiol. 2012;59:751–63. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

