Pathogen Safety of a New Intravenous Immune Globulin 10% Liquid
- PMID: 28236170
- PMCID: PMC5380692
- DOI: 10.1007/s40259-017-0212-y
Pathogen Safety of a New Intravenous Immune Globulin 10% Liquid
Abstract
Background: The manufacturing process of a new intravenous immune globulin (IVIG) 10% liquid product incorporates two dedicated pathogen safety steps: solvent/detergent (S/D) treatment and nanofiltration (20 nm). Ion-exchange chromatography (IEC) during protein purification also contributes to pathogen safety. The ability of these three process steps to inactivate/remove viruses and prions was evaluated.
Objectives: The objective of this study was to evaluate the virus and prion safety of the new IVIG 10% liquid.
Methods: Bovine viral diarrhea virus (BVDV), human immunodeficiency virus type 1 (HIV-1), mouse encephalomyelitis virus (MEV), porcine parvovirus (PPV), and pseudorabies virus (PRV) were used as models for common human viruses. The hamster-adapted scrapie strain 263K (HAS 263K) was used for transmissible spongiform encephalopathies. Virus clearance capacity and robustness of virus reduction were determined for the three steps. Abnormal prion protein (PrPSc) removal and infectivity of the samples was determined.
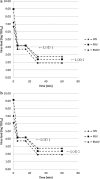
Results: S/D treatment and nanofiltration inactivated/removed enveloped viruses to below detection limits. IEC supplements viral safety and nanofiltration was highly effective in removing non-enveloped viruses and HAS 263K. Overall virus reduction factors were: ≥9.4 log10 (HIV-1), ≥13.2 log10 (PRV), ≥8.2 log10 (BVDV), ≥11.7 log10 (MEV), ≥11.6 log10 (PPV), and ≥10.4 log10 (HAS 263K).
Conclusion: Two dedicated and one supplementing steps in the manufacturing process of the new IVIG 10% liquid provide a high margin of pathogen safety.
Conflict of interest statement
Conflict of Interest
KUR, TS, GL, and JR are employees of Octapharma.
Funding
Financial support for the conduct of the research and preparation of the manuscript was provided by Octapharma.
Ethical Considerations
Pathogen safety studies were conducted in accordance with the principles of Good Laboratory Practice (Octapharma Biopharmaceuticals GmbH, Frankfurt am Main, Germany; ViruSure GmbH, Vienna, Austria), as required by the Committee for Proprietary Medicinal Products of the European Medicines Agency [14]. Animal infectivity studies were undertaken at a specialized animal facility (ViruSure). All animal procedures performed at ViruSure were approved by the Austrian Federal Ministry of Science and Research (Authorization No. BMWF-68.205/0056-II/10b/2010) and conducted according to the Austrian animal rights act (BGBL. I Nr. 169/1999) and legislative decree (BGBL II Nr.198/2000).
Figures
Similar articles
-
Biological Safety of a Highly Purified 10% Liquid Intravenous Immunoglobulin Preparation from Human Plasma.BioDrugs. 2017 Jun;31(3):251-261. doi: 10.1007/s40259-017-0222-9. BioDrugs. 2017. PMID: 28508264 Free PMC article.
-
A new liquid intravenous immunoglobulin with three dedicated virus reduction steps: virus and prion reduction capacity.Vox Sang. 2008 Apr;94(3):184-192. doi: 10.1111/j.1423-0410.2007.01016.x. Epub 2007 Dec 19. Vox Sang. 2008. PMID: 18167162
-
Removal of viruses from human intravenous immune globulin by 35 nm nanofiltration.Biologicals. 1998 Dec;26(4):321-9. doi: 10.1006/biol.1998.0164. Biologicals. 1998. PMID: 10403036
-
Pathogen safety of intravenous Rh immunoglobulin liquid and other immune globulin products: enhanced nanofiltration and manufacturing process overview.Am J Ther. 2008 Sep-Oct;15(5):435-43. doi: 10.1097/MJT.0b013e318160c1b7. Am J Ther. 2008. PMID: 18806519 Review.
-
Nanofiltration of plasma-derived biopharmaceutical products.Haemophilia. 2003 Jan;9(1):24-37. doi: 10.1046/j.1365-2516.2003.00701.x. Haemophilia. 2003. PMID: 12558776 Review.
Cited by
-
Can the administration of platelet lysates to the brain help treat neurological disorders?Cell Mol Life Sci. 2022 Jun 24;79(7):379. doi: 10.1007/s00018-022-04397-w. Cell Mol Life Sci. 2022. PMID: 35750991 Free PMC article. Review.
-
SARS-CoV-2 Neutralization in Convalescent Plasma and Commercial Lots of Plasma-Derived Immunoglobulin.BioDrugs. 2022 Jan;36(1):41-53. doi: 10.1007/s40259-021-00511-9. Epub 2021 Nov 29. BioDrugs. 2022. PMID: 34843105 Free PMC article.
-
Randomized trial of three IVIg doses for treating chronic inflammatory demyelinating polyneuropathy.Brain. 2022 Apr 29;145(3):887-896. doi: 10.1093/brain/awab422. Brain. 2022. PMID: 35038723 Free PMC article. Clinical Trial.
-
Adverse Effects of Immunoglobulin Therapy.Front Immunol. 2018 Jun 8;9:1299. doi: 10.3389/fimmu.2018.01299. eCollection 2018. Front Immunol. 2018. PMID: 29951056 Free PMC article. Review.
-
Safety and Tolerability of Intravenous Immunoglobulin in Chronic Inflammatory Demyelinating Polyneuropathy: Results of the ProCID Study.Drug Saf. 2023 Sep;46(9):835-845. doi: 10.1007/s40264-023-01326-z. Epub 2023 Jun 28. Drug Saf. 2023. PMID: 37378806 Free PMC article. Clinical Trial.
References
-
- Dwyer JM. Immunoglobulins in autoimmunity: history and mechanisms of action. Clin Exp Rheumatol. 1996;14(Suppl 15):3–7. - PubMed
-
- Ramesh S, Schwartz SA. Therapeutic uses of intravenous immunoglobulin (IVIG) in children. Pediatr Rev. 1995;16:403–410. - PubMed
-
- Orange JS, Hossny EM, Weiler CR, Ballow M, Berger M, Bonilla FA, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117:S525–S553. doi: 10.1016/j.jaci.2006.01.015. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

