Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway
- PMID: 28274999
- PMCID: PMC5739867
- DOI: 10.1136/gutjnl-2016-313075
Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway
Abstract
Objective: Neutrophils are prominent components of solid tumours and exhibit distinct phenotypes in different tumour microenvironments. However, the nature, regulation, function and clinical relevance of neutrophils in human gastric cancer (GC) are presently unknown.
Design: Flow cytometry analyses were performed to examine levels and phenotype of neutrophils in samples from 105 patients with GC. Kaplan-Meier plots for overall survival were performed using the log-rank test. Neutrophils and T cells were isolated, stimulated and/or cultured for in vitro and in vivo regulation and function assays.
Results: Patients with GC showed a significantly higher neutrophil infiltration in tumours. These tumour-infiltrating neutrophils showed an activated CD54+ phenotype and expressed high level immunosuppressive molecule programmed death-ligand 1 (PD-L1). Neutrophils activated by tumours prolonged their lifespan and strongly expressed PD-L1 proteins with similar phenotype to their status in GC, and significant correlations were found between the levels of PD-L1 and CD54 on tumour-infiltrating neutrophils. Moreover, these PD-L1+ neutrophils in tumours were associated with disease progression and reduced GC patient survival. Tumour-derived GM-CSF activated neutrophils and induced neutrophil PD-L1 expression via Janus kinase (JAK)-signal transducer and activator of transcription 3 (STAT3) signalling pathway. The activated PD-L1+ neutrophils effectively suppressed normal T-cell immunity in vitro and contributed to the growth and progression of human GC in vivo; the effect could be reversed by blocking PD-L1 on these neutrophils.
Conclusions: Our results illuminate a novel mechanism of PD-L1 expression on tumour-activated neutrophils in GC, and also provide functional evidence for these novel GM-CSF-PD-L1 pathways to prevent, and to treat this immune tolerance feature of GC.
Keywords: GASTRIC CANCER.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: None declared.
Figures


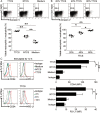


 ) or TTCS (tumour tissue culture supernatants)-conditioned neutrophils (TCN) (
) or TTCS (tumour tissue culture supernatants)-conditioned neutrophils (TCN) ( ), or TCN pre-treated with an anti-PD-L1 antibody (
), or TCN pre-treated with an anti-PD-L1 antibody ( ) or a control IgG (
) or a control IgG ( ). The illustrated data represent tumour volumes (5 mice in each group). The day of tumour cell injection was counted as day 0. *p<0.05, for groups injections with TCN pre-treated with an anti-PD-L1 antibody (
). The illustrated data represent tumour volumes (5 mice in each group). The day of tumour cell injection was counted as day 0. *p<0.05, for groups injections with TCN pre-treated with an anti-PD-L1 antibody ( ), compared with groups injections with TCN pre-treated with a control IgG (
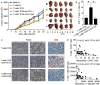
), compared with groups injections with TCN pre-treated with a control IgG ( ). The tumours were excised and photographed 24 day after injecting the tumour cells. (B and C) Interferon (IFN)-γ-producing T cell response (B) in spleens and proliferating cell nuclear antigen (PCNA) (brown) expression or CD3+ T cell infiltration (red) (C) in tumours of mice injected with T cells in combination with TCN, or TCN pre-treated with an anti-PD-L1 antibody or a control IgG on day 24 after tumour cell injection were compared. (D) The correlations between T cells and neutrophils in human tumours were analysed. Results are expressed as percentage of T cells and neutrophils in CD45+ cells or the number of T cells and neutrophils per million total cells in tumour tissues of patients with GC. Scale bars: 100 μ. Arrows indicate staining-positive cells. *p<0.05 for groups connected by horizontal lines.
). The tumours were excised and photographed 24 day after injecting the tumour cells. (B and C) Interferon (IFN)-γ-producing T cell response (B) in spleens and proliferating cell nuclear antigen (PCNA) (brown) expression or CD3+ T cell infiltration (red) (C) in tumours of mice injected with T cells in combination with TCN, or TCN pre-treated with an anti-PD-L1 antibody or a control IgG on day 24 after tumour cell injection were compared. (D) The correlations between T cells and neutrophils in human tumours were analysed. Results are expressed as percentage of T cells and neutrophils in CD45+ cells or the number of T cells and neutrophils per million total cells in tumour tissues of patients with GC. Scale bars: 100 μ. Arrows indicate staining-positive cells. *p<0.05 for groups connected by horizontal lines.
Comment in
-
Neutrophils diminish T-cell immunity to foster gastric cancer progression: the role of GM-CSF/PD-L1/PD-1 signalling pathway.Gut. 2017 Nov;66(11):1878-1880. doi: 10.1136/gutjnl-2017-313923. Epub 2017 Mar 27. Gut. 2017. PMID: 28348197 Free PMC article. No abstract available.
Similar articles
-
Granulocyte-Macrophage Colony-Stimulating Factor-Activated Neutrophils Express B7-H4 That Correlates with Gastric Cancer Progression and Poor Patient Survival.J Immunol Res. 2021 Mar 1;2021:6613247. doi: 10.1155/2021/6613247. eCollection 2021. J Immunol Res. 2021. PMID: 33763491 Free PMC article.
-
Increased intratumoral mast cells foster immune suppression and gastric cancer progression through TNF-α-PD-L1 pathway.J Immunother Cancer. 2019 Feb 26;7(1):54. doi: 10.1186/s40425-019-0530-3. J Immunother Cancer. 2019. PMID: 30808413 Free PMC article.
-
Activated neutrophils polarize protumorigenic interleukin-17A-producing T helper subsets through TNF-α-B7-H2-dependent pathway in human gastric cancer.Clin Transl Med. 2021 Jun;11(6):e484. doi: 10.1002/ctm2.484. Clin Transl Med. 2021. PMID: 34185422 Free PMC article.
-
PD-L1.J Clin Pathol. 2018 Mar;71(3):189-194. doi: 10.1136/jclinpath-2017-204853. Epub 2017 Nov 2. J Clin Pathol. 2018. PMID: 29097600 Review.
-
Programmed Death-Ligand 1 as a Regulator of Tumor Progression and Metastasis.Int J Mol Sci. 2021 May 20;22(10):5383. doi: 10.3390/ijms22105383. Int J Mol Sci. 2021. PMID: 34065396 Free PMC article. Review.
Cited by
-
The prediction potential of neutrophil-to-lymphocyte ratio for the therapeutic outcomes of programmed death receptor-1/programmed death ligand 1 inhibitors in non-small cell lung cancer patients: A meta-analysis.Medicine (Baltimore). 2020 Aug 21;99(34):e21718. doi: 10.1097/MD.0000000000021718. Medicine (Baltimore). 2020. PMID: 32846790 Free PMC article.
-
Tumor Immune Evasion Induced by Dysregulation of Erythroid Progenitor Cells Development.Cancers (Basel). 2021 Feb 19;13(4):870. doi: 10.3390/cancers13040870. Cancers (Basel). 2021. PMID: 33669537 Free PMC article. Review.
-
IL-6 regulates CCR5 expression and immunosuppressive capacity of MDSC in murine melanoma.J Immunother Cancer. 2020 Aug;8(2):e000949. doi: 10.1136/jitc-2020-000949. J Immunother Cancer. 2020. PMID: 32788238 Free PMC article.
-
Tumor-associated neutrophils upregulate Nectin2 expression, creating the immunosuppressive microenvironment in pancreatic ductal adenocarcinoma.J Exp Clin Cancer Res. 2024 Sep 11;43(1):258. doi: 10.1186/s13046-024-03178-6. J Exp Clin Cancer Res. 2024. PMID: 39261943 Free PMC article.
-
Peri-operative monocyte count is a marker of poor prognosis in gastric cancer: increased monocytes are a characteristic of myeloid-derived suppressor cells.Cancer Immunol Immunother. 2019 Aug;68(8):1341-1350. doi: 10.1007/s00262-019-02366-0. Epub 2019 Jul 19. Cancer Immunol Immunother. 2019. PMID: 31324947 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
