New knowledge of the mechanisms of sorafenib resistance in liver cancer
- PMID: 28344323
- PMCID: PMC5457690
- DOI: 10.1038/aps.2017.5
New knowledge of the mechanisms of sorafenib resistance in liver cancer
Abstract
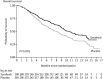
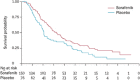
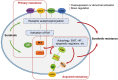
Sorafenib is an oral multikinase inhibitor that suppresses tumor cell proliferation and angiogenesis and promotes tumor cell apoptosis. It was approved by the FDA for the treatment of advanced renal cell carcinoma in 2006, and as a unique _target drug for advanced hepatocellular carcinoma (HCC) in 2007. Sorafenib can significantly extend the median survival time of patients but only by 3-5 months. Moreover, it is associated with serious adverse side effects, and drug resistance often develops. Therefore, it is of great importance to explore the mechanisms underlying sorafenib resistance and to develop individualized therapeutic strategies for coping with these problems. Recent studies have revealed that in addition to the primary resistance, several mechanisms are underlying the acquired resistance to sorafenib, such as crosstalk involving PI3K/Akt and JAK-STAT pathways, the activation of hypoxia-inducible pathways, and epithelial-mesenchymal transition. Here, we briefly describe the function of sorafenib, its clinical application, and the molecular mechanisms for drug resistance, especially for HCC patients.
Figures

Similar articles
-
Potential molecular, cellular and microenvironmental mechanism of sorafenib resistance in hepatocellular carcinoma.Cancer Lett. 2015 Oct 10;367(1):1-11. doi: 10.1016/j.canlet.2015.06.019. Epub 2015 Jul 10. Cancer Lett. 2015. PMID: 26170167 Review.
-
Dual inhibition of Akt and c-Met as a second-line therapy following acquired resistance to sorafenib in hepatocellular carcinoma cells.Mol Oncol. 2017 Mar;11(3):320-334. doi: 10.1002/1878-0261.12039. Epub 2017 Feb 17. Mol Oncol. 2017. PMID: 28164434 Free PMC article.
-
Epithelial-to-Mesenchymal Transition: A Mediator of Sorafenib Resistance in Advanced Hepatocellular Carcinoma.Curr Cancer Drug _targets. 2017;17(8):698-706. doi: 10.2174/1568009617666170427104356. Curr Cancer Drug _targets. 2017. PMID: 28460616 Review.
-
New insights into sorafenib resistance in hepatocellular carcinoma: Responsible mechanisms and promising strategies.Biochim Biophys Acta Rev Cancer. 2017 Dec;1868(2):564-570. doi: 10.1016/j.bbcan.2017.10.002. Epub 2017 Oct 17. Biochim Biophys Acta Rev Cancer. 2017. PMID: 29054475 Review.
-
Sorafenib for the treatment of advanced hepatocellular carcinoma with extrahepatic metastasis: a prospective multicenter cohort study.Cancer Med. 2015 Dec;4(12):1836-43. doi: 10.1002/cam4.548. Epub 2015 Oct 16. Cancer Med. 2015. PMID: 26471348 Free PMC article.
Cited by
-
Downregulation of MCF2L Promoted the Ferroptosis of Hepatocellular Carcinoma Cells through PI3K/mTOR Pathway in a RhoA/Rac1 Dependent Manner.Dis Markers. 2022 Oct 25;2022:6138941. doi: 10.1155/2022/6138941. eCollection 2022. Dis Markers. 2022. PMID: 36330204 Free PMC article.
-
Autophagy modulation attenuates sorafenib resistance in HCC induced in rats.Cell Death Dis. 2024 Aug 16;15(8):595. doi: 10.1038/s41419-024-06955-5. Cell Death Dis. 2024. PMID: 39152108 Free PMC article.
-
The miR-30a-5p/CLCF1 axis regulates sorafenib resistance and aerobic glycolysis in hepatocellular carcinoma.Cell Death Dis. 2020 Oct 23;11(10):902. doi: 10.1038/s41419-020-03123-3. Cell Death Dis. 2020. PMID: 33097691 Free PMC article.
-
Design, Green Synthesis and Tailoring of Vitamin E TPGS Augmented Niosomal Nano-Carrier of Pyrazolopyrimidines as Potential Anti-Liver and Breast Cancer Agents with Accentuated Oral Bioavailability.Pharmaceuticals (Basel). 2022 Mar 9;15(3):330. doi: 10.3390/ph15030330. Pharmaceuticals (Basel). 2022. PMID: 35337128 Free PMC article.
-
Systemic Treatment Options in Hepatocellular Carcinoma.Liver Cancer. 2019 Nov;8(6):427-446. doi: 10.1159/000499765. Epub 2019 May 29. Liver Cancer. 2019. PMID: 31799201 Free PMC article. Review.
References
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–90. - PubMed
-
- Blumenschein GR Jr, Gatzemeier U, Fossella F, Stewart DJ, Cupit L, Cihon F, et al. Phase II trial of single-agent sorafenib in patients with advanced non-small-cell lung carcinoma. J Clin Oncol 2006; 24: 364. - PubMed
-
- Steinbild S, Mross K, Frost A, Morant R, Gillessen S, Dittrich C, et al. A clinical phase II study with sorafenib in patients with progressive hormone-refractory prostate cancer: a study of the CESAR Central European Society for Anticancer Drug Research-EWIV. Br J Cancer 2007; 97: 1480–5. - PMC - PubMed
-
- Moreno-Aspitia A, Morton RF, Hillman DW, Lingle WL, Rowland KM Jr, Wiesenfeld M, et al. Phase II trial of sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines or taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J Clin Oncol 2009; 27: 11–5. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

