Distinct but complementary contributions of PPAR isotypes to energy homeostasis
- PMID: 28368286
- PMCID: PMC5373878
- DOI: 10.1172/JCI88894
Distinct but complementary contributions of PPAR isotypes to energy homeostasis
Abstract
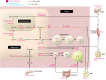
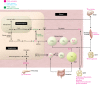
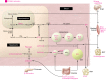
Peroxisome proliferator-activated receptors (PPARs) regulate energy metabolism and hence are therapeutic _targets in metabolic diseases such as type 2 diabetes and non-alcoholic fatty liver disease. While they share anti-inflammatory activities, the PPAR isotypes distinguish themselves by differential actions on lipid and glucose homeostasis. In this Review we discuss the complementary and distinct metabolic effects of the PPAR isotypes together with the underlying cellular and molecular mechanisms, as well as the synthetic PPAR ligands that are used in the clinic or under development. We highlight the potential of new PPAR ligands with improved efficacy and safety profiles in the treatment of complex metabolic disorders.
Conflict of interest statement
Figures
Similar articles
-
Peroxisome Proliferator-Activated Receptors and Their Novel Ligands as Candidates for the Treatment of Non-Alcoholic Fatty Liver Disease.Cells. 2020 Jul 8;9(7):1638. doi: 10.3390/cells9071638. Cells. 2020. PMID: 32650421 Free PMC article. Review.
-
PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD.Nat Rev Endocrinol. 2017 Jan;13(1):36-49. doi: 10.1038/nrendo.2016.135. Epub 2016 Sep 16. Nat Rev Endocrinol. 2017. PMID: 27636730 Review.
-
Anti-NASH Drug Development Hitches a Lift on PPAR Agonism.Cells. 2019 Dec 21;9(1):37. doi: 10.3390/cells9010037. Cells. 2019. PMID: 31877771 Free PMC article. Review.
-
A review of the studies on food-derived factors which regulate energy metabolism via the modulation of lipid-sensing nuclear receptors.Biosci Biotechnol Biochem. 2019 Apr;83(4):579-588. doi: 10.1080/09168451.2018.1559025. Epub 2018 Dec 20. Biosci Biotechnol Biochem. 2019. PMID: 30572788 Review.
-
Role of peroxisome proliferator-activated receptors in non-alcoholic fatty liver disease inflammation.Cell Mol Life Sci. 2018 Aug;75(16):2951-2961. doi: 10.1007/s00018-018-2838-4. Epub 2018 May 22. Cell Mol Life Sci. 2018. PMID: 29789866 Free PMC article. Review.
Cited by
-
The Role of PPARγ Gene Polymorphisms, Gut Microbiota in Type 2 Diabetes: Current Progress and Future Prospects.Diabetes Metab Syndr Obes. 2023 Nov 7;16:3557-3566. doi: 10.2147/DMSO.S429825. eCollection 2023. Diabetes Metab Syndr Obes. 2023. PMID: 37954888 Free PMC article. Review.
-
Peroxisome Proliferator-Activated Receptors and Their Novel Ligands as Candidates for the Treatment of Non-Alcoholic Fatty Liver Disease.Cells. 2020 Jul 8;9(7):1638. doi: 10.3390/cells9071638. Cells. 2020. PMID: 32650421 Free PMC article. Review.
-
PPARs as Nuclear Receptors for Nutrient and Energy Metabolism.Molecules. 2019 Jul 12;24(14):2545. doi: 10.3390/molecules24142545. Molecules. 2019. PMID: 31336903 Free PMC article. Review.
-
A Case of Infantile Alagille Syndrome With Severe Dyslipidemia: New Insight into Lipid Metabolism and Therapeutics.J Endocr Soc. 2022 Jan 18;6(3):bvac005. doi: 10.1210/jendso/bvac005. eCollection 2022 Mar 1. J Endocr Soc. 2022. PMID: 35155971 Free PMC article.
-
Characterizing 3T3-L1 MBX Adipocyte Cell Differentiation Maintained with Fatty Acids as an In Vitro Model to Study the Effects of Obesity.Life (Basel). 2023 Aug 9;13(8):1712. doi: 10.3390/life13081712. Life (Basel). 2023. PMID: 37629569 Free PMC article.
References
-
- Gross B, Pawlak M, Lefebvre P, Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol. 2017;13(1):36–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

