Protective effect of TM6 on LPS-induced acute lung injury in mice
- PMID: 28373694
- PMCID: PMC5428560
- DOI: 10.1038/s41598-017-00551-8
Protective effect of TM6 on LPS-induced acute lung injury in mice
Abstract
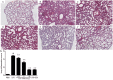
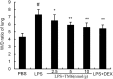
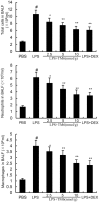
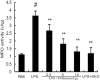
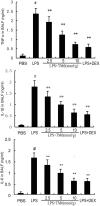
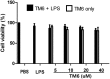
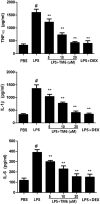
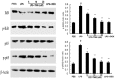
Acute lung injury (ALI) is an acute failure of the respiratory system for which effective treatment is urgently necessary. Previous studies found that several peptides potently inhibited the production of cytokines induced by lipopolysaccharide (LPS). In this study, we synthetized a cell-permeable TIR domain-derived decoy peptide (TM6) and examined its substance for the ability to inhibit TLR signaling in the model of ALI induced by LPS. We demonstrated that TM6 (2.5, 5 and 10 nmol/g) alleviated the histological changes in the lung tissues as well as myeloperoxtidase (MPO) activity, lung W/D ratio, the production of TNF-α, IL-1β and IL-6 induced by LPS. Furthermore, the numbers of total cells, neutrophils and macrophages in the BALF were suppressed by TM6. In vitro, TM6 (5, 10 and 20 µM) inhibited the production of TNF-α, IL-1β and IL-6 in LPS-stimulated alveolar macrophages. Moreover, the activation of Nuclear factor-kappaB (NF-κB) and Mitogen activated protein kinases (MAPK) signaling pathways induced by LPS were also inhibited by TM6. Collectively, our results suggested that TM6 was an effective inhibitor of ALI induced by LPS, and this peptide may very well serve as a future treatment for ALI.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Acanthoic acid ameliorates lipopolysaccharide-induced acute lung injury.Eur J Pharmacol. 2015 Mar 5;750:32-8. doi: 10.1016/j.ejphar.2015.01.023. Epub 2015 Jan 23. Eur J Pharmacol. 2015. PMID: 25620130
-
Ugonin M, a Helminthostachys zeylanica Constituent, Prevents LPS-Induced Acute Lung Injury through TLR4-Mediated MAPK and NF-κB Signaling Pathways.Molecules. 2017 Apr 1;22(4):573. doi: 10.3390/molecules22040573. Molecules. 2017. PMID: 28368327 Free PMC article.
-
Picrasma quassiodes (D. Don) Benn. attenuates lipopolysaccharide (LPS)-induced acute lung injury.Int J Mol Med. 2016 Sep;38(3):834-44. doi: 10.3892/ijmm.2016.2669. Epub 2016 Jul 7. Int J Mol Med. 2016. PMID: 27431288
-
Gastrodin protects against LPS-induced acute lung injury by activating Nrf2 signaling pathway.Onco_target. 2017 May 9;8(19):32147-32156. doi: 10.18632/onco_target.16740. Onco_target. 2017. PMID: 28389632 Free PMC article.
-
Ouabain Protects Mice Against Lipopolysaccharide-Induced Acute Lung Injury.Med Sci Monit. 2018 Jun 28;24:4455-4464. doi: 10.12659/MSM.908627. Med Sci Monit. 2018. PMID: 29953424 Free PMC article.
Cited by
-
Periplaneta Americana Extract Ameliorates LPS-induced Acute Lung Injury Via Reducing Inflammation and Oxidative Stress.Curr Med Sci. 2023 Jun;43(3):445-455. doi: 10.1007/s11596-023-2723-8. Epub 2023 May 16. Curr Med Sci. 2023. PMID: 37191939
-
Na/K-ATPase suppresses LPS-induced pro-inflammatory signaling through Lyn.iScience. 2022 Aug 17;25(9):104963. doi: 10.1016/j.isci.2022.104963. eCollection 2022 Sep 16. iScience. 2022. PMID: 36072548 Free PMC article.
-
Aspirin eugenol ester alleviates lipopolysaccharide-induced acute lung injury in rats while stabilizing serum metabolites levels.Front Immunol. 2022 Jul 29;13:939106. doi: 10.3389/fimmu.2022.939106. eCollection 2022. Front Immunol. 2022. PMID: 35967416 Free PMC article.
-
Efficacy of CU06-1004 via regulation of inflammation and endothelial permeability in LPS-induced acute lung injury.J Inflamm (Lond). 2023 Apr 6;20(1):13. doi: 10.1186/s12950-023-00338-x. J Inflamm (Lond). 2023. PMID: 37024954 Free PMC article.
-
Beyond Transduction: Anti-Inflammatory Effects of Cell Penetrating Peptides.Molecules. 2024 Aug 29;29(17):4088. doi: 10.3390/molecules29174088. Molecules. 2024. PMID: 39274936 Free PMC article. Review.
References
-
- Epidemiology of transfusion related acute lung injury in Gifit, the French hemovigilance database. A study of the French hemovigilance network. Vox Sang89, 8–8 (2005).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

