The phosphatidylinositol-3-phosphate 5-kinase inhibitor apilimod blocks filoviral entry and infection
- PMID: 28403145
- PMCID: PMC5402990
- DOI: 10.1371/journal.pntd.0005540
The phosphatidylinositol-3-phosphate 5-kinase inhibitor apilimod blocks filoviral entry and infection
Abstract
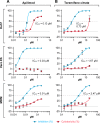
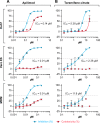
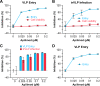
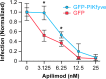
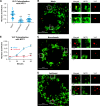
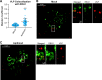
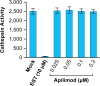
Phosphatidylinositol-3-phosphate 5-kinase (PIKfyve) is a lipid kinase involved in endosome maturation that emerged from a haploid genetic screen as being required for Ebola virus (EBOV) infection. Here we analyzed the effects of apilimod, a PIKfyve inhibitor that was reported to be well tolerated in humans in phase 2 clinical trials, for its effects on entry and infection of EBOV and Marburg virus (MARV). We first found that apilimod blocks infections by EBOV and MARV in Huh 7, Vero E6 and primary human macrophage cells, with notable potency in the macrophages (IC50, 10 nM). We next observed that similar doses of apilimod block EBOV-glycoprotein-virus like particle (VLP) entry and transcription-replication competent VLP infection, suggesting that the primary mode of action of apilimod is as an entry inhibitor, preventing release of the viral genome into the cytoplasm to initiate replication. After providing evidence that the anti-EBOV action of apilimod is via PIKfyve, we showed that it blocks trafficking of EBOV VLPs to endolysosomes containing Niemann-Pick C1 (NPC1), the intracellular receptor for EBOV. Concurrently apilimod caused VLPs to accumulate in early endosome antigen 1-positive endosomes. We did not detect any effects of apilimod on bulk endosome acidification, on the activity of cathepsins B and L, or on cholesterol export from endolysosomes. Hence by antagonizing PIKfyve, apilimod appears to block EBOV trafficking to its site of fusion and entry into the cytoplasm. Given the drug's observed anti-filoviral activity, relatively unexplored mechanism of entry inhibition, and reported tolerability in humans, we propose that apilimod be further explored as part of a therapeutic regimen to treat filoviral infections.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Inhibition of Ebola and Marburg Virus Entry by G Protein-Coupled Receptor Antagonists.J Virol. 2015 Oct;89(19):9932-8. doi: 10.1128/JVI.01337-15. Epub 2015 Jul 22. J Virol. 2015. PMID: 26202243 Free PMC article.
-
Ebola virus triggers receptor tyrosine kinase-dependent signaling to promote the delivery of viral particles to entry-conducive intracellular compartments.PLoS Pathog. 2021 Jan 29;17(1):e1009275. doi: 10.1371/journal.ppat.1009275. eCollection 2021 Jan. PLoS Pathog. 2021. PMID: 33513206 Free PMC article.
-
Multiple cationic amphiphiles induce a Niemann-Pick C phenotype and inhibit Ebola virus entry and infection.PLoS One. 2013;8(2):e56265. doi: 10.1371/journal.pone.0056265. Epub 2013 Feb 18. PLoS One. 2013. PMID: 23441171 Free PMC article.
-
Potential pharmacological strategies _targeting the Niemann-Pick C1 receptor and Ebola virus glycoprotein interaction.Eur J Med Chem. 2021 Nov 5;223:113654. doi: 10.1016/j.ejmech.2021.113654. Epub 2021 Jun 19. Eur J Med Chem. 2021. PMID: 34175537 Review.
-
Development of treatment strategies to combat Ebola and Marburg viruses.Expert Rev Anti Infect Ther. 2006 Feb;4(1):67-76. doi: 10.1586/14787210.4.1.67. Expert Rev Anti Infect Ther. 2006. PMID: 16441210 Review.
Cited by
-
A PIKfyve modulator combined with an integrated stress response inhibitor to treat lysosomal storage diseases.Proc Natl Acad Sci U S A. 2024 Aug 20;121(34):e2320257121. doi: 10.1073/pnas.2320257121. Epub 2024 Aug 16. Proc Natl Acad Sci U S A. 2024. PMID: 39150784
-
Inhibition of Ebola Virus by a Molecularly Engineered Banana Lectin.PLoS Negl Trop Dis. 2019 Jul 29;13(7):e0007595. doi: 10.1371/journal.pntd.0007595. eCollection 2019 Jul. PLoS Negl Trop Dis. 2019. PMID: 31356611 Free PMC article.
-
PIP4K2C inhibition reverses autophagic flux impairment induced by SARS-CoV-2.bioRxiv [Preprint]. 2024 Apr 17:2024.04.15.589676. doi: 10.1101/2024.04.15.589676. bioRxiv. 2024. PMID: 38659941 Free PMC article. Preprint.
-
Beyond PI3Ks: _targeting phosphoinositide kinases in disease.Nat Rev Drug Discov. 2023 May;22(5):357-386. doi: 10.1038/s41573-022-00582-5. Epub 2022 Nov 14. Nat Rev Drug Discov. 2023. PMID: 36376561 Free PMC article. Review.
-
Neuropathophysiology of coronavirus disease 2019: neuroinflammation and blood brain barrier disruption are critical pathophysiological processes that contribute to the clinical symptoms of SARS-CoV-2 infection.Inflammopharmacology. 2021 Aug;29(4):939-963. doi: 10.1007/s10787-021-00806-x. Epub 2021 Apr 6. Inflammopharmacology. 2021. PMID: 33822324 Free PMC article. Review.
References
-
- La Vega de M-A, Stein D, Kobinger GP. Ebolavirus Evolution: Past and Present. PLoS Pathog. 2015;11: e1005221 doi: 10.1371/journal.ppat.1005221 - DOI - PMC - PubMed
-
- Ebola Situation Report. 2016 Mar.
-
- Qiu X, Wong G, Audet J, Bello A, Fernando L, Alimonti JB, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514: 47–53. doi: 10.1038/nature13777 - DOI - PMC - PubMed
-
- Misasi J, Gilman MSA, Kanekiyo M, Gui M, Cagigi A, Mulangu S, et al. Structural and molecular basis for Ebola virus neutralization by protective human antibodies. Science. American Association for the Advancement of Science; 2016;351: 1343–1346. doi: 10.1126/science.aad6117 - DOI - PMC - PubMed
-
- Corti D, Misasi J, Mulangu S, Stanley DA, Kanekiyo M, Wollen S, et al. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science. American Association for the Advancement of Science; 2016;351: 1339–1342. doi: 10.1126/science.aad5224 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

