Thermodynamics in cancers: opposing interactions between PPAR gamma and the canonical WNT/beta-catenin pathway
- PMID: 28405929
- PMCID: PMC5389954
- DOI: 10.1186/s40169-017-0144-7
Thermodynamics in cancers: opposing interactions between PPAR gamma and the canonical WNT/beta-catenin pathway
Abstract
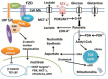
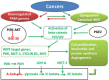
Cancer cells are the site of numerous metabolic and thermodynamic abnormalities. We focus this review on the interactions between the canonical WNT/beta-catenin pathway and peroxisome proliferator-activated receptor gamma (PPAR gamma) in cancers and their implications from an energetic and metabolic point of view. In numerous tissues, PPAR gamma activation induces inhibition of beta-catenin pathway, while the activation of the canonical WNT/beta-catenin pathway inactivates PPAR gamma. In most cancers but not all, PPAR gamma is downregulated while the WNT/beta-catenin pathway is upregulated. In cancer cells, upregulation of the WNT/beta-catenin signaling induces dramatic changes in key metabolic enzymes that modify their thermodynamic behavior. This leads to activation of pyruvate dehydrogenase kinase1 (PDK-1) and monocarboxylate lactate transporter. Consequently, phosphorylation of PDK-1 inhibits the pyruvate dehydrogenase complex (PDH). Thus, a large part of pyruvate cannot be converted into acetyl-coenzyme A (acetyl-CoA) in mitochondria and only a part of acetyl-CoA can enter the tricarboxylic acid cycle. This leads to aerobic glycolysis in spite of the availability of oxygen. This phenomenon is referred to as the Warburg effect. Cytoplasmic pyruvate is converted into lactate. The WNT/beta-catenin pathway induces the transcription of genes involved in cell proliferation, i.e., MYC and CYCLIN D1. This ultimately promotes the nucleotide, protein and lipid synthesis necessary for cell growth and multiplication. In cancer, activation of the PI3K-AKT pathway induces an increase of the aerobic glycolysis. Moreover, prostaglandin E2 by activating the canonical WNT pathway plays also a role in cancer. In addition in many cancer cells, PPAR gamma is downregulated. Moreover, PPAR gamma contributes to regulate some key circadian genes. In cancers, abnormalities in the regulation of circadian rhythms (CRs) are observed. CRs are dissipative structures which play a key-role in far-from-equilibrium thermodynamics. In cancers, metabolism, thermodynamics and CRs are intimately interrelated.
Keywords: Aerobic glycolysis; Cancer; Circadian rhythms; Dissipative structures; PI3 K-AKT pathway; PPAR gamma; Pyruvate dehydrogenase complex; Pyruvate dehydrogenase kinase; WNT/beta-catenin; Warburg effect.
Figures
Similar articles
-
Thermodynamics in Gliomas: Interactions between the Canonical WNT/Beta-Catenin Pathway and PPAR Gamma.Front Physiol. 2017 May 30;8:352. doi: 10.3389/fphys.2017.00352. eCollection 2017. Front Physiol. 2017. PMID: 28620312 Free PMC article. Review.
-
Thermodynamics in Neurodegenerative Diseases: Interplay Between Canonical WNT/Beta-Catenin Pathway-PPAR Gamma, Energy Metabolism and Circadian Rhythms.Neuromolecular Med. 2018 Jun;20(2):174-204. doi: 10.1007/s12017-018-8486-x. Epub 2018 Mar 23. Neuromolecular Med. 2018. PMID: 29572723 Review.
-
Interactions between PPAR Gamma and the Canonical Wnt/Beta-Catenin Pathway in Type 2 Diabetes and Colon Cancer.PPAR Res. 2017;2017:5879090. doi: 10.1155/2017/5879090. Epub 2017 Feb 19. PPAR Res. 2017. PMID: 28298922 Free PMC article. Review.
-
Aerobic Glycolysis Hypothesis Through WNT/Beta-Catenin Pathway in Exudative Age-Related Macular Degeneration.J Mol Neurosci. 2017 Aug;62(3-4):368-379. doi: 10.1007/s12031-017-0947-4. Epub 2017 Jul 8. J Mol Neurosci. 2017. PMID: 28689265 Review.
-
Demyelination in Multiple Sclerosis: Reprogramming Energy Metabolism and Potential PPARγ Agonist Treatment Approaches.Int J Mol Sci. 2018 Apr 16;19(4):1212. doi: 10.3390/ijms19041212. Int J Mol Sci. 2018. PMID: 29659554 Free PMC article. Review.
Cited by
-
Multiple _targets of the Canonical WNT/β-Catenin Signaling in Cancers.Front Oncol. 2019 Nov 18;9:1248. doi: 10.3389/fonc.2019.01248. eCollection 2019. Front Oncol. 2019. PMID: 31803621 Free PMC article. Review.
-
Circadian and Metabolic Perspectives in the Role Played by NADPH in Cancer.Front Endocrinol (Lausanne). 2018 Mar 15;9:93. doi: 10.3389/fendo.2018.00093. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29599747 Free PMC article. Review.
-
Crosstalk Between Peroxisome Proliferator-Activated Receptor Gamma and the Canonical WNT/β-Catenin Pathway in Chronic Inflammation and Oxidative Stress During Carcinogenesis.Front Immunol. 2018 Apr 13;9:745. doi: 10.3389/fimmu.2018.00745. eCollection 2018. Front Immunol. 2018. PMID: 29706964 Free PMC article. Review.
-
The role of peroxisome proliferator-activated receptors in the tumor microenvironment, tumor cell metabolism, and anticancer therapy.Front Pharmacol. 2023 May 12;14:1184794. doi: 10.3389/fphar.2023.1184794. eCollection 2023. Front Pharmacol. 2023. PMID: 37251321 Free PMC article. Review.
-
The Emerging Role of PPAR Beta/Delta in Tumor Angiogenesis.PPAR Res. 2020 Aug 13;2020:3608315. doi: 10.1155/2020/3608315. eCollection 2020. PPAR Res. 2020. PMID: 32855630 Free PMC article. Review.
References
-
- Schrödinger E (1944) What is life?: Cambridge University Press, Cambridge
-
- Atkins PW. Physical chemistry. Oxford: Oxford University Press; 1990. pp. 1–1031.
-
- Kondepudi D, Prigogine I. Modern thermodynamics from heat engines to dissipative structures. New York: Wiley; 1999. pp. 1–486.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
