Endocannabinoid system acts as a regulator of immune homeostasis in the gut
- PMID: 28439004
- PMCID: PMC5441729
- DOI: 10.1073/pnas.1612177114
Endocannabinoid system acts as a regulator of immune homeostasis in the gut
Abstract
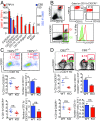
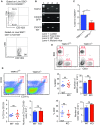
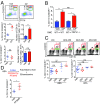
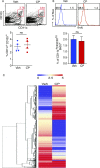
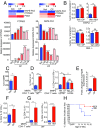
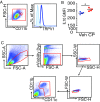
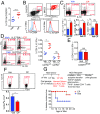
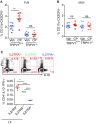
Endogenous cannabinoids (endocannabinoids) are small molecules biosynthesized from membrane glycerophospholipid. Anandamide (AEA) is an endogenous intestinal cannabinoid that controls appetite and energy balance by engagement of the enteric nervous system through cannabinoid receptors. Here, we uncover a role for AEA and its receptor, cannabinoid receptor 2 (CB2), in the regulation of immune tolerance in the gut and the pancreas. This work demonstrates a major immunological role for an endocannabinoid. The pungent molecule capsaicin (CP) has a similar effect as AEA; however, CP acts by engagement of the vanilloid receptor TRPV1, causing local production of AEA, which acts through CB2. We show that the engagement of the cannabinoid/vanilloid receptors augments the number and immune suppressive function of the regulatory CX3CR1hi macrophages (Mϕ), which express the highest levels of such receptors among the gut immune cells. Additionally, TRPV1-/- or CB2-/- mice have fewer CX3CR1hi Mϕ in the gut. Treatment of mice with CP also leads to differentiation of a regulatory subset of CD4+ cells, the Tr1 cells, in an IL-27-dependent manner in vitro and in vivo. In a functional demonstration, tolerance elicited by engagement of TRPV1 can be transferred to naïve nonobese diabetic (NOD) mice [model of type 1 diabetes (T1D)] by transfer of CD4+ T cells. Further, oral administration of AEA to NOD mice provides protection from T1D. Our study unveils a role for the endocannabinoid system in maintaining immune homeostasis in the gut/pancreas and reveals a conversation between the nervous and immune systems using distinct receptors.
Keywords: CX3CR1 macrophage; T-regulatory cells; cannabis; diabetes; mucosal immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Characterization of the endocannabinoid system in human spermatozoa and involvement of transient receptor potential vanilloid 1 receptor in their fertilizing ability.Endocrinology. 2009 Oct;150(10):4692-700. doi: 10.1210/en.2009-0057. Epub 2009 Jul 16. Endocrinology. 2009. PMID: 19608651
-
Modulation of trigeminal sensory neuron activity by the dual cannabinoid-vanilloid agonists anandamide, N-arachidonoyl-dopamine and arachidonyl-2-chloroethylamide.Br J Pharmacol. 2004 Apr;141(7):1118-30. doi: 10.1038/sj.bjp.0705711. Epub 2004 Mar 8. Br J Pharmacol. 2004. PMID: 15006899 Free PMC article.
-
Anandamide induces matrix metalloproteinase-2 production through cannabinoid-1 receptor and transient receptor potential vanilloid-1 in human dental pulp cells in culture.J Endod. 2012 Jun;38(6):786-90. doi: 10.1016/j.joen.2012.02.025. Epub 2012 Apr 11. J Endod. 2012. PMID: 22595113
-
Cannabinoids and the gut: new developments and emerging concepts.Pharmacol Ther. 2010 Apr;126(1):21-38. doi: 10.1016/j.pharmthera.2009.12.005. Epub 2010 Feb 1. Pharmacol Ther. 2010. PMID: 20117132 Review.
-
Endocannabinoids in immune regulation and immunopathologies.Immunology. 2021 Oct;164(2):242-252. doi: 10.1111/imm.13378. Epub 2021 Jul 8. Immunology. 2021. PMID: 34053085 Free PMC article. Review.
Cited by
-
Effects of Cannabidiol Chewing Gum on Perceived Pain and Well-Being of Irritable Bowel Syndrome Patients: A Placebo-Controlled Crossover Exploratory Intervention Study with Symptom-Driven Dosing.Cannabis Cannabinoid Res. 2022 Aug;7(4):436-444. doi: 10.1089/can.2020.0087. Epub 2021 Feb 11. Cannabis Cannabinoid Res. 2022. PMID: 33998882 Free PMC article. Clinical Trial.
-
Critical roles of G protein-coupled receptors in regulating intestinal homeostasis and inflammatory bowel disease.Mucosal Immunol. 2022 May;15(5):819-828. doi: 10.1038/s41385-022-00538-3. Epub 2022 Jun 22. Mucosal Immunol. 2022. PMID: 35732818 Review.
-
The Immune Endocannabinoid System of the Tumor Microenvironment.Int J Mol Sci. 2020 Nov 25;21(23):8929. doi: 10.3390/ijms21238929. Int J Mol Sci. 2020. PMID: 33255584 Free PMC article. Review.
-
Medical Cannabis and Cannabinoids: An Option for the Treatment of Inflammatory Bowel Disease and Cancer of the Colon?Med Cannabis Cannabinoids. 2018 Jun 12;1(1):28-35. doi: 10.1159/000489036. eCollection 2018 Jun. Med Cannabis Cannabinoids. 2018. PMID: 34676319 Free PMC article. Review.
-
Novel approaches to the treatment of type 1 diabetes.J Diabetes. 2022 Nov;14(11):724-726. doi: 10.1111/1753-0407.13333. Epub 2022 Nov 7. J Diabetes. 2022. PMID: 36345147 Free PMC article. No abstract available.
References
-
- McPartland JM, Matias I, Di Marzo V, Glass M. Evolutionary origins of the endocannabinoid system. Gene. 2006;370:64–74. - PubMed
-
- Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004;3:771–784. - PubMed
-
- Schmid HH, Schmid PC, Natarajan V. N-acylated glycerophospholipids and their derivatives. Prog Lipid Res. 1990;29:1–43. - PubMed
-
- Schmid HH. Pathways and mechanisms of N-acylethanolamine biosynthesis: Can anandamide be generated selectively? Chem Phys Lipids. 2000;108:71–87. - PubMed
-
- Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005;8:585–589. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

