DNA-PK Promotes the Mitochondrial, Metabolic, and Physical Decline that Occurs During Aging
- PMID: 28467930
- PMCID: PMC5485859
- DOI: 10.1016/j.cmet.2017.04.008
DNA-PK Promotes the Mitochondrial, Metabolic, and Physical Decline that Occurs During Aging
Erratum in
-
DNA-PK Promotes the Mitochondrial, Metabolic, and Physical Decline that Occurs During Aging.Cell Metab. 2017 Aug 1;26(2):447. doi: 10.1016/j.cmet.2017.07.005. Cell Metab. 2017. PMID: 28768182 No abstract available.
Abstract
Hallmarks of aging that negatively impact health include weight gain and reduced physical fitness, which can increase insulin resistance and risk for many diseases, including type 2 diabetes. The underlying mechanism(s) for these phenomena is poorly understood. Here we report that aging increases DNA breaks and activates DNA-dependent protein kinase (DNA-PK) in skeletal muscle, which suppresses mitochondrial function, energy metabolism, and physical fitness. DNA-PK phosphorylates threonines 5 and 7 of HSP90α, decreasing its chaperone function for clients such as AMP-activated protein kinase (AMPK), which is critical for mitochondrial biogenesis and energy metabolism. Decreasing DNA-PK activity increases AMPK activity and prevents weight gain, decline of mitochondrial function, and decline of physical fitness in middle-aged mice and protects against type 2 diabetes. In conclusion, DNA-PK is one of the drivers of the metabolic and fitness decline during aging, and therefore DNA-PK inhibitors may have therapeutic potential in obesity and low exercise capacity.
Keywords: AMPK; DNA-PK; HSP90α; aging; calorie restriction; exercise; insulin sensitivity; mitochondria; obesity; skeletal muscle; type 2 diabetes.
Published by Elsevier Inc.
Figures
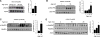


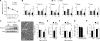
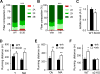
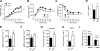
Comment in
-
Turning back the clock.Sci Transl Med. 2017 May 17;9(390):eaan4290. doi: 10.1126/scitranslmed.aan4290. Sci Transl Med. 2017. PMID: 28515340
Similar articles
-
SIRT6 regulates metabolic homeostasis in skeletal muscle through activation of AMPK.Am J Physiol Endocrinol Metab. 2017 Oct 1;313(4):E493-E505. doi: 10.1152/ajpendo.00122.2017. Epub 2017 Aug 1. Am J Physiol Endocrinol Metab. 2017. PMID: 28765271
-
Exercise training attenuates aging-associated mitochondrial dysfunction in rat skeletal muscle: role of PGC-1α.Exp Gerontol. 2013 Nov;48(11):1343-50. doi: 10.1016/j.exger.2013.08.004. Epub 2013 Aug 30. Exp Gerontol. 2013. PMID: 23994518
-
Astaxanthin stimulates mitochondrial biogenesis in insulin resistant muscle via activation of AMPK pathway.J Cachexia Sarcopenia Muscle. 2020 Feb;11(1):241-258. doi: 10.1002/jcsm.12530. Epub 2020 Jan 31. J Cachexia Sarcopenia Muscle. 2020. PMID: 32003547 Free PMC article.
-
The role of weight loss and exercise in correcting skeletal muscle mitochondrial abnormalities in obesity, diabetes and aging.Mol Cell Endocrinol. 2013 Oct 15;379(1-2):30-4. doi: 10.1016/j.mce.2013.06.018. Epub 2013 Jun 20. Mol Cell Endocrinol. 2013. PMID: 23792186 Review.
-
The role of AMPK in controlling metabolism and mitochondrial biogenesis during exercise.Exp Physiol. 2014 Dec 1;99(12):1581-5. doi: 10.1113/expphysiol.2014.082255. Epub 2014 Sep 25. Exp Physiol. 2014. PMID: 25261498 Review.
Cited by
-
DNA-PKcs promotes sepsis-induced multiple organ failure by triggering mitochondrial dysfunction.J Adv Res. 2022 Nov;41:39-48. doi: 10.1016/j.jare.2022.01.014. Epub 2022 Jan 31. J Adv Res. 2022. PMID: 36328752 Free PMC article.
-
In situ observation of mitochondrial biogenesis as the early event of apoptosis.iScience. 2021 Aug 27;24(9):103038. doi: 10.1016/j.isci.2021.103038. eCollection 2021 Sep 24. iScience. 2021. PMID: 34553131 Free PMC article.
-
Inherent aerobic capacity-dependent differences in breast carcinogenesis.Carcinogenesis. 2017 Sep 1;38(9):920-928. doi: 10.1093/carcin/bgx066. Carcinogenesis. 2017. PMID: 28911004 Free PMC article.
-
The Ku complex: recent advances and emerging roles outside of non-homologous end-joining.Cell Mol Life Sci. 2021 May;78(10):4589-4613. doi: 10.1007/s00018-021-03801-1. Epub 2021 Apr 15. Cell Mol Life Sci. 2021. PMID: 33855626 Free PMC article. Review.
-
DNA-Dependent Protein Kinase Catalytic Subunit (DNA-PKcs): Beyond the DNA Double-Strand Break Repair.Mol Cells. 2023 Apr 30;46(4):200-205. doi: 10.14348/molcells.2023.2164. Epub 2023 Feb 9. Mol Cells. 2023. PMID: 36756777 Free PMC article. Review.
References
-
- Anderson CW, Carter TH. The DNA-activated protein kinase – DNA-PK. Curr Top Microbiol Immunol. 1996;217:91–111. - PubMed
-
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. - PubMed
-
- Barazzoni R, Short KR, Nair KS. Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J Biol Chem. 2000;275:3343–3347. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

