Lysosomal enzyme replacement therapies: Historical development, clinical outcomes, and future perspectives
- PMID: 28502768
- PMCID: PMC5828774
- DOI: 10.1016/j.addr.2017.05.004
Lysosomal enzyme replacement therapies: Historical development, clinical outcomes, and future perspectives
Abstract
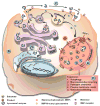
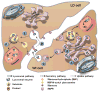
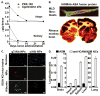
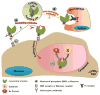
Lysosomes and lysosomal enzymes play a central role in numerous cellular processes, including cellular nutrition, recycling, signaling, defense, and cell death. Genetic deficiencies of lysosomal components, most commonly enzymes, are known as "lysosomal storage disorders" or "lysosomal diseases" (LDs) and lead to lysosomal dysfunction. LDs broadly affect peripheral organs and the central nervous system (CNS), debilitating patients and frequently causing fatality. Among other approaches, enzyme replacement therapy (ERT) has advanced to the clinic and represents a beneficial strategy for 8 out of the 50-60 known LDs. However, despite its value, current ERT suffers from several shortcomings, including various side effects, development of "resistance", and suboptimal delivery throughout the body, particularly to the CNS, lowering the therapeutic outcome and precluding the use of this strategy for a majority of LDs. This review offers an overview of the biomedical causes of LDs, their socio-medical relevance, treatment modalities and caveats, experimental alternatives, and future treatment perspectives.
Keywords: Blood-brain barrier delivery; Enzyme carriers; Enzyme replacement therapy; Enzyme _targeting and delivery; ICAM-1 mediated enzyme delivery; Lysosomal diseases; Lysosomal enzyme deficiency; Lysosomal storage disorders; Multi-organ dysfunction; Neurodegeneration; Side effects.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Advances in therapies for neurological lysosomal storage disorders.J Inherit Metab Dis. 2023 Sep;46(5):874-905. doi: 10.1002/jimd.12615. Epub 2023 May 2. J Inherit Metab Dis. 2023. PMID: 37078180 Review.
-
New Advanced Strategies for the Treatment of Lysosomal Diseases Affecting the Central Nervous System.Curr Pharm Des. 2019;25(17):1933-1950. doi: 10.2174/1381612825666190708213159. Curr Pharm Des. 2019. PMID: 31566121 Review.
-
Comparative binding, endocytosis, and biodistribution of antibodies and antibody-coated carriers for _targeted delivery of lysosomal enzymes to ICAM-1 versus transferrin receptor.J Inherit Metab Dis. 2013 May;36(3):467-77. doi: 10.1007/s10545-012-9534-6. Epub 2012 Sep 12. J Inherit Metab Dis. 2013. PMID: 22968581 Free PMC article.
-
Therapeutic approaches for lysosomal storage diseases: a patent update.Recent Pat CNS Drug Discov. 2013 Aug;8(2):91-109. doi: 10.2174/15748898113089990002. Recent Pat CNS Drug Discov. 2013. PMID: 23713988 Review.
-
Enzyme Replacement Therapy: A Review and Its Role in Treating Lysosomal Storage Diseases.Pediatr Ann. 2018 May 1;47(5):e191-e197. doi: 10.3928/19382359-20180424-01. Pediatr Ann. 2018. PMID: 29750286 Review.
Cited by
-
Dissociation of 19F and fluorescence signal upon cellular uptake of dual-contrast perfluorocarbon nanoemulsions.MAGMA. 2019 Feb;32(1):133-145. doi: 10.1007/s10334-018-0723-7. Epub 2018 Nov 29. MAGMA. 2019. PMID: 30498884
-
Strategies for Enhancing the Permeation of CNS-Active Drugs through the Blood-Brain Barrier: A Review.Molecules. 2018 May 28;23(6):1289. doi: 10.3390/molecules23061289. Molecules. 2018. PMID: 29843371 Free PMC article. Review.
-
δ-Tocopherol Effect on Endocytosis and Its Combination with Enzyme Replacement Therapy for Lysosomal Disorders: A New Type of Drug Interaction?J Pharmacol Exp Ther. 2019 Sep;370(3):823-833. doi: 10.1124/jpet.119.257345. Epub 2019 May 17. J Pharmacol Exp Ther. 2019. PMID: 31101681 Free PMC article.
-
Therapeutic Approaches in Lysosomal Storage Diseases.Biomolecules. 2021 Nov 26;11(12):1775. doi: 10.3390/biom11121775. Biomolecules. 2021. PMID: 34944420 Free PMC article. Review.
-
Neuronal Ceroid Lipofuscinosis: Potential for _targeted Therapy.Drugs. 2021 Jan;81(1):101-123. doi: 10.1007/s40265-020-01440-7. Drugs. 2021. PMID: 33242182 Review.
References
-
- Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nature reviews Molecular cell biology. 2007;8:622–632. - PubMed
-
- Abeliovich A, Gitler AD. Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature. 2016;539:207–216. - PubMed
-
- Futerman AH, van Meer G. The cell biology of lysosomal storage disorders. Nature reviews Molecular cell biology. 2004;5:554–565. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

