Genomic and non-genomic effects of androgens in the cardiovascular system: clinical implications
- PMID: 28645930
- PMCID: PMC5736922
- DOI: 10.1042/CS20170090
Genomic and non-genomic effects of androgens in the cardiovascular system: clinical implications
Abstract
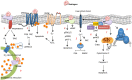
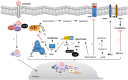
The principle steroidal androgens are testosterone and its metabolite 5α-dihydrotestosterone (DHT), which is converted from testosterone by the enzyme 5α-reductase. Through the classic pathway with androgens crossing the plasma membrane and binding to the androgen receptor (AR) or via mechanisms independent of the ligand-dependent transactivation function of nuclear receptors, testosterone induces genomic and non-genomic effects respectively. AR is widely distributed in several tissues, including vascular endothelial and smooth muscle cells. Androgens are essential for many developmental and physiological processes, especially in male reproductive tissues. It is now clear that androgens have multiple actions besides sex differentiation and sexual maturation and that many physiological systems are influenced by androgens, including regulation of cardiovascular function [nitric oxide (NO) release, Ca2+ mobilization, vascular apoptosis, hypertrophy, calcification, senescence and reactive oxygen species (ROS) generation]. This review focuses on evidence indicating that interplay between genomic and non-genomic actions of testosterone may influence cardiovascular function.
Keywords: androgen receptor; cardiovascular; genomic; non-genomic.
© 2017 The Author(s). published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
Similar articles
-
The role of non-aromatizable testosterone metabolite in metabolic pathways.Physiol Res. 2011;60(2):253-61. doi: 10.33549/physiolres.932080. Epub 2010 Nov 29. Physiol Res. 2011. PMID: 21114370 Review.
-
Prolactin influences upon androgen action in male accessory sex organs.Adv Sex Horm Res. 1976;2:425-70. Adv Sex Horm Res. 1976. PMID: 189591 Review.
-
Reactive oxygen species: players in the cardiovascular effects of testosterone.Am J Physiol Regul Integr Comp Physiol. 2016 Jan 1;310(1):R1-14. doi: 10.1152/ajpregu.00392.2014. Epub 2015 Nov 4. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 26538238 Free PMC article. Review.
-
Mechanisms and clinical relevance of androgens and androgen receptor actions.Chang Gung Med J. 2003 Jun;26(6):388-402. Chang Gung Med J. 2003. PMID: 12956285 Review.
-
Androgens are not major down-regulators of androgen receptor levels during growth of the immature rat penis.J Steroid Biochem Mol Biol. 1996 Mar;57(5-6):301-13. doi: 10.1016/0960-0760(95)00283-9. J Steroid Biochem Mol Biol. 1996. PMID: 8639466
Cited by
-
Vascular Pathways of Testosterone: Clinical Implications.J Cardiovasc Transl Res. 2020 Feb;13(1):55-72. doi: 10.1007/s12265-019-09939-5. Epub 2019 Dec 9. J Cardiovasc Transl Res. 2020. PMID: 31820333 Review.
-
Battle of the sexes: contrasting roles of testis-specific protein Y-encoded (TSPY) and TSPX in human oncogenesis.Asian J Androl. 2019 May-Jun;21(3):260-269. doi: 10.4103/aja.aja_43_18. Asian J Androl. 2019. PMID: 29974883 Free PMC article. Review.
-
Vitamin D Deficiency Reduces Vascular Reactivity of Coronary Arterioles in Male Rats.Curr Issues Mol Biol. 2021 May 7;43(1):79-92. doi: 10.3390/cimb43010007. Curr Issues Mol Biol. 2021. PMID: 34066967 Free PMC article.
-
Testosterone, β-estradiol, and hepatocellular carcinoma: stimulation or inhibition? A comparative effect analysis on cell cycle, apoptosis, and Wnt signaling of HepG2 cells.Naunyn Schmiedebergs Arch Pharmacol. 2024 Aug;397(8):6121-6133. doi: 10.1007/s00210-024-03019-5. Epub 2024 Feb 29. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38421409
-
Gender-Affirming Hormone Therapy, Vascular Health and Cardiovascular Disease in Transgender Adults.Hypertension. 2019 Dec;74(6):1266-1274. doi: 10.1161/HYPERTENSIONAHA.119.13080. Epub 2019 Oct 28. Hypertension. 2019. PMID: 31656099 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

