Nasal administration of the neuroprotective candidate NeuroEPO to healthy volunteers: a randomized, parallel, open-label safety study
- PMID: 28676085
- PMCID: PMC5496637
- DOI: 10.1186/s12883-017-0908-0
Nasal administration of the neuroprotective candidate NeuroEPO to healthy volunteers: a randomized, parallel, open-label safety study
Abstract
Background: Delivery of therapeutic agents as erythropoietin (EPO) into Central Nervous System through intranasal route could benefit patients with neurological disorders. A new nasal formulation containing a non-hematopoietic recombinant EPO (NeuroEPO) has shown neuroprotective actions in preclinical models. In the current study, the safety of NeuroEPO was evaluated for the first time in humans.
Methods: A phase I, randomized, parallel, open-label study was carried out in healthy volunteers. They received, intranasally, 1 mg of NeuroEPO every 8 h during 4 days (Group A) or 0.5 mg of NeuroEPO (Group B) with the same schedule. The working hypothesis was that intranasal NeuroEPO produce <10% of severe adverse reactions in the evaluated groups. Therefore, a rigorous assessment of possible adverse events was carried out, which included tolerance of the nasal mucosa and the effect on hematopoietic activity. Clinical safety evaluation was daily during treatment and laboratory tests were done before and on days 5 and 14 after starting treatment.
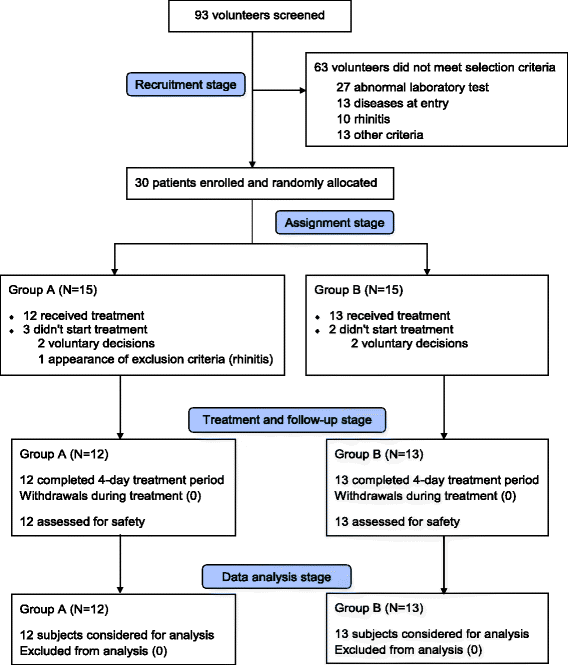
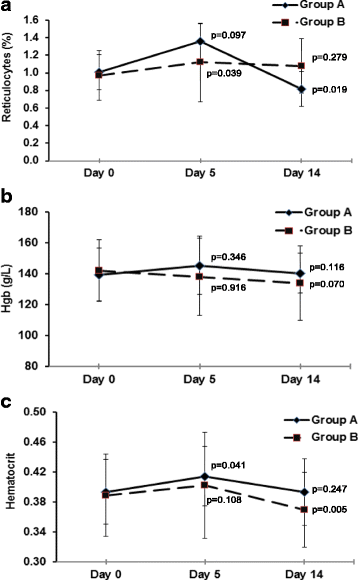
Results: Twenty-five volunteers, 56% women, with a mean age of 27 yrs. were included. Twelve of them received the highest NeuroEPO dose. Twenty types of adverse events occurred, with headache (20%) and increase of hepatic enzymes (20%) as the most reported ones. Nasopharyngeal itching was the most common local event but only observed in four patients (16%), all of them from the lowest dose group. About half of the events were very probably or probably caused by the studied product. Most of the events were mild (95.5%), did not require treatment (88.6%) and were completely resolved (81.8%). No severe adverse events were reported. During the study the hematopoietic variables were kept within reference values.
Conclusions: NeuroEPO was a safe product, well tolerated at the nasal mucosa level and did not stimulate erythropoiesis in healthy volunteers.
Trial registration: Cuban Public Registry of Clinical Trials RPCEC00000157 , June 10, 2013.
Keywords: Healthy volunteers; Hematopoietic activity; NeuroEPO; Neurodegenerative diseases; Non-hematopoietic recombinant erythropoietin; Safety; Stroke.
Conflict of interest statement
Ethics approval and consent to participate
The clinical protocol was approved by the institutional ethics committee of the National Center for Toxicology in Havana. All procedures performed in healthy volunteers were in accordance with the ethical standards of this committee and with the 1964 Helsinki declaration and its later amendments. The trial was also approved by the Cuban Center for the Control of Drugs, Equipment & Medical Devices (reference number: 05.018.13.B). Prior to any test, all volunteers gave their written informed consent to participate.
Consent for publication
Not applicable.
Competing interests
Authors OSM, YPI, CMG, PPS and DAG are employees of the Center of Molecular Immunology (CIM), Havana, Cuba, where NeuroEPO intranasal formulation is produced. Authors DJR, TFC and IGG are employees of the Center for Drug Research and Development (CIDEM), Havana, Cuba, where this formulation was developed and partly preclinically developed. Drs. García-Rodríguez and Sosa-Teste are co-inventors of the patent of rHu-EPO nasal formulations (see ref. [14]). The rest of the authors have no competing interests concerning this paper.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Short-term Tolerance of Nasally-administered NeuroEPO in Patients with Parkinson Disease.MEDICC Rev. 2021 Jan;23(1):49-54. doi: 10.37757/MR2021.V23.N1.10. Epub 2021 Jan 30. MEDICC Rev. 2021. PMID: 33780423 Clinical Trial.
-
Recombinant human erythropoietin in the treatment of acute ischemic stroke.Stroke. 2009 Dec;40(12):e647-56. doi: 10.1161/STROKEAHA.109.564872. Epub 2009 Oct 15. Stroke. 2009. PMID: 19834012 Clinical Trial.
-
NeuroEPO Preserves Neurons from Glutamate-Induced Excitotoxicity.J Alzheimers Dis. 2018;65(4):1469-1483. doi: 10.3233/JAD-180668. J Alzheimers Dis. 2018. PMID: 30175978
-
The nasal route as a potential pathway for delivery of erythropoietin in the treatment of acute ischemic stroke in humans.ScientificWorldJournal. 2009 Sep 15;9:970-81. doi: 10.1100/tsw.2009.103. ScientificWorldJournal. 2009. PMID: 19768354 Free PMC article. Review.
-
Intranasal erythropoietin therapy in nervous system disorders.Expert Opin Drug Deliv. 2011 Jan;8(1):19-32. doi: 10.1517/17425247.2011.540236. Epub 2010 Dec 13. Expert Opin Drug Deliv. 2011. PMID: 21143002 Review.
Cited by
-
Neuroimmunology Research. A Report from the Cuban Network of Neuroimmunology.Behav Sci (Basel). 2018 May 8;8(5):47. doi: 10.3390/bs8050047. Behav Sci (Basel). 2018. PMID: 29738432 Free PMC article.
-
The Effect of Erythropoietin and Its Derivatives on Ischemic Stroke Therapy: A Comprehensive Review.Front Pharmacol. 2022 Feb 17;13:743926. doi: 10.3389/fphar.2022.743926. eCollection 2022. Front Pharmacol. 2022. PMID: 35250554 Free PMC article. Review.
-
Erythropoietin and Friedreich Ataxia: Time for a Reappraisal?Front Neurosci. 2019 Apr 24;13:386. doi: 10.3389/fnins.2019.00386. eCollection 2019. Front Neurosci. 2019. PMID: 31105516 Free PMC article. Review.
-
_targeting for Success: Demonstrating Proof-of-Concept with Mechanistic Early Phase Clinical Pharmacology Studies for Disease-Modification in Neurodegenerative Disorders.Int J Mol Sci. 2021 Feb 5;22(4):1615. doi: 10.3390/ijms22041615. Int J Mol Sci. 2021. PMID: 33562713 Free PMC article. Review.
-
Non-Invasive Strategies for Nose-to-Brain Drug Delivery.J Clin Trials. 2020;10(7):439. Epub 2020 Dec 10. J Clin Trials. 2020. PMID: 33505777 Free PMC article.
References
-
- Wang YL, Liang H, Song SL. The intervention treatment of neuroprotection for ischemic stroke. Sheng Li Ke Xue Jin Zhan. 2012;43:279–282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

