Endothelial cell SHP-2 negatively regulates neutrophil adhesion and promotes transmigration by enhancing ICAM-1-VE-cadherin interaction
- PMID: 28701303
- PMCID: PMC5636709
- DOI: 10.1096/fj.201700280R
Endothelial cell SHP-2 negatively regulates neutrophil adhesion and promotes transmigration by enhancing ICAM-1-VE-cadherin interaction
Abstract
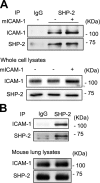
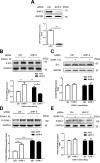
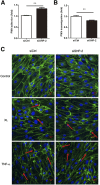
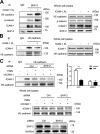
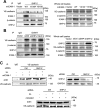
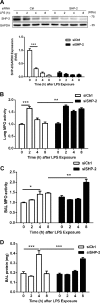
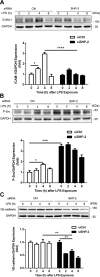
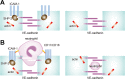
Intercellular adhesion molecule-1 (ICAM-1) mediates the firm adhesion of leukocytes to endothelial cells and initiates subsequent signaling that promotes their transendothelial migration (TEM). Vascular endothelial (VE)-cadherin plays a critical role in endothelial cell-cell adhesion, thereby controlling endothelial permeability and leukocyte transmigration. This study aimed to determine the molecular signaling events that originate from the ICAM-1-mediated firm adhesion of neutrophils that regulate VE-cadherin's role as a negative regulator of leukocyte transmigration. We observed that ICAM-1 interacts with Src homology domain 2-containing phosphatase-2 (SHP-2), and SHP-2 down-regulation via silencing of small interfering RNA in endothelial cells enhanced neutrophil adhesion to endothelial cells but inhibited neutrophil transmigration. We also found that VE-cadherin associated with the ICAM-1-SHP-2 complex. Moreover, whereas the activation of ICAM-1 leads to VE-cadherin dissociation from ICAM-1 and VE-cadherin association with actin, SHP-2 down-regulation prevented ICAM-1-VE-cadherin association and promoted VE-cadherin-actin association. Furthermore, SHP-2 down-regulation in vivo promoted LPS-induced neutrophil recruitment in mouse lung but delayed neutrophil extravasation. These results suggest that SHP-2-via association with ICAM-1-mediates ICAM-1-induced Src activation and modulates VE-cadherin switching association with ICAM-1 or actin, thereby negatively regulating neutrophil adhesion to endothelial cells and enhancing their TEM.-Yan, M., Zhang, X., Chen, A., Gu, W., Liu, J., Ren, X., Zhang, J., Wu, X., Place, A. T., Minshall, R. D., Liu, G. Endothelial cell SHP-2 negatively regulates neutrophil adhesion and promotes transmigration by enhancing ICAM-1-VE-cadherin interaction.
Keywords: LPS; inflammation; leukocyte; lung injury.
© FASEB.
Figures
Similar articles
-
Csk controls leukocyte extravasation via local regulation of Src family kinases and cortactin signaling.Front Immunol. 2024 Oct 28;15:1480152. doi: 10.3389/fimmu.2024.1480152. eCollection 2024. Front Immunol. 2024. PMID: 39530094 Free PMC article.
-
ICAM-1-activated Src and eNOS signaling increase endothelial cell surface PECAM-1 adhesivity and neutrophil transmigration.Blood. 2012 Aug 30;120(9):1942-52. doi: 10.1182/blood-2011-12-397430. Epub 2012 Jul 17. Blood. 2012. PMID: 22806890 Free PMC article.
-
ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration.J Immunol. 2007 Sep 15;179(6):4053-64. doi: 10.4049/jimmunol.179.6.4053. J Immunol. 2007. PMID: 17785844
-
Dynamic Regulation of Vascular Permeability by Vascular Endothelial Cadherin-Mediated Endothelial Cell-Cell Junctions.J Nippon Med Sch. 2017;84(4):148-159. doi: 10.1272/jnms.84.148. J Nippon Med Sch. 2017. PMID: 28978894 Review.
-
Neutrophil recruitment under shear flow: it's all about endothelial cell rings and gaps.Microcirculation. 2009 Jan;16(1):43-57. doi: 10.1080/10739680802273892. Microcirculation. 2009. PMID: 18720226 Free PMC article. Review.
Cited by
-
Periostin+ cancer-associated fibroblasts promote lymph node metastasis by impairing the lymphatic endothelial barriers in cervical squamous cell carcinoma.Mol Oncol. 2021 Jan;15(1):210-227. doi: 10.1002/1878-0261.12837. Epub 2020 Nov 12. Mol Oncol. 2021. PMID: 33124726 Free PMC article.
-
Effects of 2',6'-dihydroxy-4'-methoxydihidrochalcone on innate inflammatory response.Naunyn Schmiedebergs Arch Pharmacol. 2020 Nov;393(11):2061-2072. doi: 10.1007/s00210-020-01922-1. Epub 2020 Jun 16. Naunyn Schmiedebergs Arch Pharmacol. 2020. PMID: 32548784
-
Tripterin liposome relieves severe acute respiratory syndrome as a potent COVID-19 treatment.Signal Transduct _target Ther. 2022 Dec 24;7(1):399. doi: 10.1038/s41392-022-01283-6. Signal Transduct _target Ther. 2022. PMID: 36566328 Free PMC article.
-
Small molecule compound M12 reduces vascular permeability in obese mice via blocking endothelial TRPV4-Nox2 interaction.Acta Pharmacol Sin. 2022 Jun;43(6):1430-1440. doi: 10.1038/s41401-021-00780-8. Epub 2021 Oct 15. Acta Pharmacol Sin. 2022. PMID: 34654876 Free PMC article.
-
Neoplastic ICAM-1 protects lung carcinoma from apoptosis through ligation of fibrinogen.Cell Death Dis. 2024 Aug 21;15(8):605. doi: 10.1038/s41419-024-06989-9. Cell Death Dis. 2024. PMID: 39168965 Free PMC article.
References
-
- Wallez Y., Cand F., Cruzalegui F., Wernstedt C., Souchelnytskyi S., Vilgrain I., Huber P. (2007) Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique _target site. Oncogene. 26, 1067–1077 - PubMed
-
- Wessel F., Winderlich M., Holm M., Frye M., Rivera-Galdos R., Vockel M., Linnepe R., Ipe U., Stadtmann A., Zarbock A., Nottebaum A. F., Vestweber D. (2014) Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nat. Immunol. 15, 223–230 - PubMed
-
- Giannotta M., Trani M., Dejana E. (2013) VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev. Cell 26, 441–454 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

