Impact of neonatal hypoxia-ischaemia on oligodendrocyte survival, maturation and myelinating potential
- PMID: 28782169
- PMCID: PMC5742723
- DOI: 10.1111/jcmm.13309
Impact of neonatal hypoxia-ischaemia on oligodendrocyte survival, maturation and myelinating potential
Abstract
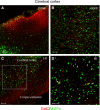
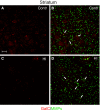
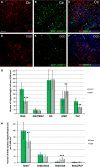
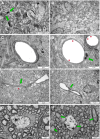
Hypoxic-ischaemic episodes experienced at the perinatal period commonly lead to a development of neurological disabilities and cognitive impairments in neonates or later in childhood. Clinical symptoms often are associated with the observed alterations in white matter in the brains of diseased children, suggesting contribution of triggered oligodendrocyte/myelin pathology to the resulting disorders. To date, the processes initiated by perinatal asphyxia remain unclear, hampering the ability to develop preventions. To address the issue, the effects of temporal hypoxia-ischaemia on survival, proliferation and the myelinating potential of oligodendrocytes were evaluated ex vivo using cultures of hippocampal organotypic slices and in vivo in rat model of perinatal asphyxia. The potential engagement of gelatinases in oligodendrocyte maturation was assessed as well. The results pointed to a significant decrease in the number of oligodendrocyte progenitor cells (OPCs), which is compensated for to a certain extent by the increased rate of OPC proliferation. Oligodendrocyte maturation seemed however to be significantly altered. An ultrastructural examination of selected brain regions performed several weeks after the insult showed however that the process of developing central nervous system myelination proceeds efficiently resulting in enwrapping the majority of axons in compact myelin. The increased angiogenesis in response to neonatal hypoxic-ischaemic insult was also noticed. In conclusion, the study shows that hypoxic-ischaemic episodes experienced during the most active period of nervous system development might be efficiently compensated for by the oligodendroglial cell response triggered by the insult. The main obstacle seems to be the inflammatory process modulating the local microenvironment.
Keywords: electron microscopy; gelatinases; hippocampal organotypic slices; myelin structure; myelinogenesis; neonatal hypoxia-ischaemia; oligodendrocyte progenitor cells; oxygen and glycose deprivation; perinatal asphyxia.
© 2017 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures



Similar articles
-
Oligodendrocyte progenitor cells' fate after neonatal asphyxia-Puzzling implications for the development of hypoxic-ischemic encephalopathy.Brain Pathol. 2024 Nov;34(6):e13255. doi: 10.1111/bpa.13255. Epub 2024 Mar 19. Brain Pathol. 2024. PMID: 38504469 Free PMC article.
-
Oligodendrocyte Response to Pathophysiological Conditions Triggered by Episode of Perinatal Hypoxia-Ischemia: Role of IGF-1 Secretion by Glial Cells.Mol Neurobiol. 2020 Oct;57(10):4250-4268. doi: 10.1007/s12035-020-02015-z. Epub 2020 Jul 21. Mol Neurobiol. 2020. PMID: 32691304 Free PMC article.
-
Effects of hypothermia on oligodendrocyte precursor cell proliferation, differentiation and maturation following hypoxia ischemia in vivo and in vitro.Exp Neurol. 2013 Sep;247:720-9. doi: 10.1016/j.expneurol.2013.03.015. Epub 2013 Mar 22. Exp Neurol. 2013. PMID: 23524193
-
Therapeutic Strategies for Leukodystrophic Disorders Resulting from Perinatal Asphyxia: Focus on Myelinating Oligodendrocytes.Mol Neurobiol. 2018 May;55(5):4388-4402. doi: 10.1007/s12035-017-0647-7. Epub 2017 Jun 28. Mol Neurobiol. 2018. PMID: 28660484 Free PMC article. Review.
-
Myelin and oligodendrocyte lineage cells in white matter pathology and plasticity after traumatic brain injury.Neuropharmacology. 2016 Nov;110(Pt B):654-659. doi: 10.1016/j.neuropharm.2015.04.029. Epub 2015 May 9. Neuropharmacology. 2016. PMID: 25963414 Review.
Cited by
-
Impact of a Histone Deacetylase Inhibitor-Trichostatin A on Neurogenesis after Hypoxia-Ischemia in Immature Rats.Int J Mol Sci. 2020 May 27;21(11):3808. doi: 10.3390/ijms21113808. Int J Mol Sci. 2020. PMID: 32471267 Free PMC article.
-
Neuron-glia Interaction as a Possible Pathophysiological Mechanism of Bipolar Disorder.Curr Neuropharmacol. 2018;16(5):519-532. doi: 10.2174/1570159X15666170828170921. Curr Neuropharmacol. 2018. PMID: 28847296 Free PMC article. Review.
-
Neonatal Mesenchymal Stem Cell Treatment Improves Myelination Impaired by Global Perinatal Asphyxia in Rats.Int J Mol Sci. 2021 Mar 23;22(6):3275. doi: 10.3390/ijms22063275. Int J Mol Sci. 2021. PMID: 33806988 Free PMC article.
-
Effect of the HDAC Inhibitor, Sodium Butyrate, on Neurogenesis in a Rat Model of Neonatal Hypoxia-Ischemia: Potential Mechanism of Action.Mol Neurobiol. 2019 Sep;56(9):6341-6370. doi: 10.1007/s12035-019-1518-1. Epub 2019 Feb 14. Mol Neurobiol. 2019. PMID: 30767185 Free PMC article.
-
Intermittent theta-burst stimulation alleviates hypoxia-ischemia-caused myelin damage and neurologic disability.Exp Neurol. 2024 Aug;378:114821. doi: 10.1016/j.expneurol.2024.114821. Epub 2024 May 21. Exp Neurol. 2024. PMID: 38782349
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

