Influence of Layer-by-Layer Polyelectrolyte Deposition and EDC/NHS Activated Heparin Immobilization onto Silk Fibroin Fabric
- PMID: 28788601
- PMCID: PMC5453351
- DOI: 10.3390/ma7042956
Influence of Layer-by-Layer Polyelectrolyte Deposition and EDC/NHS Activated Heparin Immobilization onto Silk Fibroin Fabric
Abstract
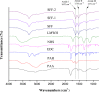
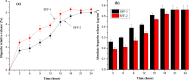
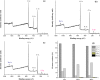
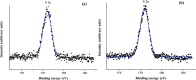
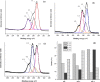
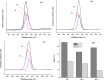
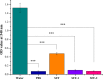
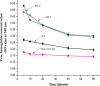
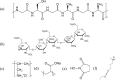
To enhance the hemocompatibility of silk fibroin fabric as biomedical material, polyelectrolytes architectures have been assembled through the layer-by-layer (LbL) technique on silk fibroin fabric (SFF). In particular, 1.5 and 2.5 bilayer of oppositely charged polyelectrolytes were assembled onto SFF using poly(allylamine hydrochloride) (PAH) as polycationic polymer and poly(acrylic acid) (PAA) as polyanionic polymer with PAH topmost. Low molecular weight heparin (LMWH) activated with 1-ethyl-3-(dimethylaminopropyl) carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) was then immobilized on its surface. Alcian Blue staining, toluidine blue assay and X-ray photoelectron spectroscopy (XPS) confirmed the presence of heparin on modified SFF surfaces. The surface morphology of the modified silk fibroin fabric surfaces was characterized by scanning electron microscopy (SEM) and atomic force microscopy (AFM), and obtained increased roughness. Negligible hemolytic effect and a higher concentration of free hemoglobin by a kinetic clotting time test ensured the improved biological performance of the modified fibroin fabric. Overall, the deposition of 2.5 bilayer was found effective in terms of biological and surface properties of the modified fibroin fabric compared to 1.5 bilayer self-assembly technique. Therefore, this novel approach to surface modification may demonstrate long term patency in future in vivo animal trials of small diameter silk fibroin vascular grafts.
Keywords: EDC/NHS; hemocompatibility; heparin; layer-by-layer; low molecular weight heparin (LMWH); poly(acrylic acid); poly(allylamine hydrochloride); silk fibroin fabric.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Surface modification of silk fibroin fabric using layer-by-layer polyelectrolyte deposition and heparin immobilization for small-diameter vascular prostheses.Langmuir. 2015 Mar 3;31(8):2517-26. doi: 10.1021/la504503w. Epub 2015 Feb 20. Langmuir. 2015. PMID: 25671295
-
Surface Modification and Characterisation of Silk Fibroin Fabric Produced by the Layer-by-Layer Self-Assembly of Multilayer Alginate/Regenerated Silk Fibroin.PLoS One. 2015 Apr 28;10(4):e0124811. doi: 10.1371/journal.pone.0124811. eCollection 2015. PLoS One. 2015. PMID: 25919690 Free PMC article.
-
Dual-functional composite with anticoagulant and antibacterial properties based on heparinized silk fibroin and chitosan.Colloids Surf B Biointerfaces. 2011 Jul 1;85(2):241-7. doi: 10.1016/j.colsurfb.2011.02.035. Epub 2011 Mar 8. Colloids Surf B Biointerfaces. 2011. PMID: 21459560
-
Silk fibroin coating through EDC/NHS crosslink is an effective method to promote graft remodeling of a polyethylene terephthalate artificial ligament.J Biomater Appl. 2019 May;33(10):1407-1414. doi: 10.1177/0885328219836625. Epub 2019 Mar 18. J Biomater Appl. 2019. PMID: 30885033
-
Surface Modification with Particles Coated or Made of Polymer Multilayers.Pharmaceutics. 2022 Nov 16;14(11):2483. doi: 10.3390/pharmaceutics14112483. Pharmaceutics. 2022. PMID: 36432674 Free PMC article. Review.
Cited by
-
Development and Characterization of a Fucoidan-Based Drug Delivery System by Using Hydrophilic Anticancer Polysaccharides to Simultaneously Deliver Hydrophobic Anticancer Drugs.Biomolecules. 2020 Jun 28;10(7):970. doi: 10.3390/biom10070970. Biomolecules. 2020. PMID: 32605162 Free PMC article.
-
Facile Modification of NF Membrane by Multi-Layer Deposition of Polyelectrolytes for Enhanced Fouling Resistance.Polymers (Basel). 2021 Oct 28;13(21):3728. doi: 10.3390/polym13213728. Polymers (Basel). 2021. PMID: 34771283 Free PMC article.
-
The Interactions of Quantum Dot-Labeled Silk Fibroin Micro/Nanoparticles with Cells.Materials (Basel). 2020 Jul 30;13(15):3372. doi: 10.3390/ma13153372. Materials (Basel). 2020. PMID: 32751473 Free PMC article.
-
Immunosensing for Early Detection of Rheumatoid Arthritis Biomarkers: Anti-Cyclic Citrullinated Peptide Antibodies Based on Tilted-Fiber Bragg Grating Biosensor.Bioengineering (Basel). 2023 Feb 16;10(2):261. doi: 10.3390/bioengineering10020261. Bioengineering (Basel). 2023. PMID: 36829755 Free PMC article.
-
A biomimetic approach to modulating the sustained release of fibroblast growth factor 2 from fibrin microthread scaffolds.Explor Biomat X. 2024;1(2):58-83. doi: 10.37349/ebmx.2024.00006. Epub 2024 Apr 23. Explor Biomat X. 2024. PMID: 39070763 Free PMC article.
References
-
- Bae J.-S., Seo E.-J., Kang I.-K. Synthesis and characterization of heparinized polyurethanes using plasma glow discharge. Biomaterials. 1999;20:529–537. - PubMed
-
- Klement P., Du Y.J., Berry L., Andrew M., Chan A.K.C. Blood-compatible biomaterials by surface coating with a novel antithrombin–heparin covalent complex. Biomaterials. 2002;23:527–535. - PubMed
-
- Babatasi G., Massetti M., Bara L., Mazmanian M., Samama M., Khayat A. Graft thrombosis in small diameter vascular prosthesis: A laboratory model. Int. J. Angiol. 1997;6:118–123.
-
- Baguneid M.S., Seifalian A.M., Salacinski H.J., Murray D., Hamilton G., Walker M.G. Tissue engineering of blood vessels. Br. J. Surg. 2006;93:282–290. - PubMed
-
- Kannan R.Y., Salacinski H.J., Edirisinghe M.J., Hamilton G., Seifalian A.M. Polyhedral oligomeric silsequioxane-polyurethane nanocomposite microvessels for an artificial capillary bed. Biomaterials. 2006;27:4618–4626. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

