Telomere biology and telomerase mutations in cirrhotic patients with hepatocellular carcinoma
- PMID: 28813500
- PMCID: PMC5558955
- DOI: 10.1371/journal.pone.0183287
Telomere biology and telomerase mutations in cirrhotic patients with hepatocellular carcinoma
Abstract
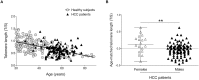
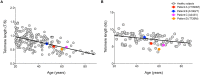
Telomeres are repetitive DNA sequences at linear chromosome termini, protecting chromosomes against end-to-end fusion and damage, providing chromosomal stability. Telomeres shorten with mitotic cellular division, but are maintained in cells with high proliferative capacity by telomerase. Loss-of-function mutations in telomere-maintenance genes are genetic risk factors for cirrhosis development in humans and murine models. Telomerase deficiency provokes accelerated telomere shortening and dysfunction, facilitating genomic instability and oncogenesis. Here we examined whether telomerase mutations and telomere shortening were associated with hepatocellular carcinoma (HCC) secondary to cirrhosis. Telomere length of peripheral blood leukocytes was measured by Southern blot and qPCR in 120 patients with HCC associated with cirrhosis and 261 healthy subjects. HCC patients were screened for telomerase gene variants (in TERT and TERC) by Sanger sequencing. Age-adjusted telomere length was comparable between HCC patients and healthy subjects by both Southern blot and qPCR. Four non-synonymous TERT heterozygous variants were identified in four unrelated patients, resulting in a significantly higher mutation carrier frequency (3.3%) in patients as compared to controls (p = 0.02). Three of the four variants (T726M, A1062T, and V1090M) were previously observed in patients with other telomere diseases (severe aplastic anemia, acute myeloid leukemia, and cirrhosis). A novel TERT variant, A243V, was identified in a 65-year-old male with advanced HCC and cirrhosis secondary to chronic hepatitis C virus (HCV) and alcohol ingestion, but direct assay measurements in vitro did not detect modulation of telomerase enzymatic activity or processivity. In summary, constitutional variants resulting in amino acid changes in the telomerase reverse transcriptase were found in a small proportion of patients with cirrhosis-associated HCC.
Conflict of interest statement
Figures

Similar articles
-
Telomerase activity, telomere length and human telomerase reverse transcriptase expression in hepatocellular carcinoma is independent of hepatitis virus status.Liver Int. 2009 Sep;29(8):1162-70. doi: 10.1111/j.1478-3231.2009.02082.x. Epub 2009 Jul 17. Liver Int. 2009. PMID: 19627485
-
Progressive telomere shortening and telomerase reactivation during hepatocellular carcinogenesis.Cancer Genet Cytogenet. 1997 Jan;93(1):56-62. doi: 10.1016/s0165-4608(96)00329-9. Cancer Genet Cytogenet. 1997. PMID: 9062581
-
Telomere length is key to hepatocellular carcinoma diversity and telomerase addiction is an actionable therapeutic _target.J Hepatol. 2021 May;74(5):1155-1166. doi: 10.1016/j.jhep.2020.11.052. Epub 2020 Dec 15. J Hepatol. 2021. PMID: 33338512
-
Telomere and telomerase in chronic liver disease and hepatocarcinoma.World J Gastroenterol. 2014 May 28;20(20):6287-92. doi: 10.3748/wjg.v20.i20.6287. World J Gastroenterol. 2014. PMID: 24876749 Free PMC article. Review.
-
Telomeres and telomerase: new _targets for the treatment of liver cirrhosis and hepatocellular carcinoma.J Hepatol. 2004 Sep;41(3):491-7. doi: 10.1016/j.jhep.2004.06.010. J Hepatol. 2004. PMID: 15336455 Review. No abstract available.
Cited by
-
Studying the pathogenicity of 26 variants characterized in the first molecular analyses of Egyptian aplastic anemia patients.J Genet Eng Biotechnol. 2023 Nov 29;21(1):149. doi: 10.1186/s43141-023-00585-8. J Genet Eng Biotechnol. 2023. PMID: 38017244 Free PMC article.
-
Telomerase Expression Related with Poor Immune Response to HCV Core Antigen in Egyptian HCV Patients' PBMCs.J Clin Exp Hepatol. 2023 Nov-Dec;13(6):1008-1016. doi: 10.1016/j.jceh.2023.06.004. Epub 2023 Jun 19. J Clin Exp Hepatol. 2023. PMID: 37975051 Free PMC article.
-
Genetic Counseling and Family Screening Recommendations in Patients with Telomere Biology Disorders.Curr Hematol Malig Rep. 2023 Dec;18(6):273-283. doi: 10.1007/s11899-023-00713-8. Epub 2023 Oct 3. Curr Hematol Malig Rep. 2023. PMID: 37787873 Review.
-
Recent advances in understanding telomere diseases.Fac Rev. 2022 Oct 19;11:31. doi: 10.12703/r/11-31. eCollection 2022. Fac Rev. 2022. PMID: 36311538 Free PMC article. Review.
-
Fanconi anemia and dyskeratosis congenita/telomere biology disorders: Two inherited bone marrow failure syndromes with genomic instability.Front Oncol. 2022 Aug 25;12:949435. doi: 10.3389/fonc.2022.949435. eCollection 2022. Front Oncol. 2022. PMID: 36091172 Free PMC article. Review.
References
-
- Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106(6):661–73. . - PubMed
-
- Blasco MA. Telomere length, stem cells and aging. Nat Chem Biol. 2007;3(10):640–9. doi: 10.1038/nchembio.2007.38 . - DOI - PubMed
-
- Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361(24):2353–65. doi: 10.1056/NEJMra0903373 . - DOI - PMC - PubMed
-
- Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43(2 Pt 1):405–13. . - PubMed
-
- Lingner J, Hughes TR, Shevchenko A, Mann M, Lundblad V, Cech TR. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science. 1997;276(5312):561–7. . - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

