Novel Textile Scaffolds Generated by Flock Technology for Tissue Engineering of Bone and Cartilage
- PMID: 28817062
- PMCID: PMC5448925
- DOI: 10.3390/ma5030540
Novel Textile Scaffolds Generated by Flock Technology for Tissue Engineering of Bone and Cartilage
Abstract
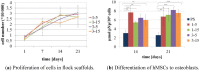
Textile scaffolds can be found in a variety of application areas in regenerative medicine and tissue engineering. In the present study we used electrostatic flocking-a well-known textile technology-to produce scaffolds for tissue engineering of bone. Flock scaffolds stand out due to their unique structure: parallel arranged fibers that are aligned perpendicularly to a substrate, resulting in mechanically stable structures with a high porosity. In compression tests we demonstrated good mechanical properties of such scaffolds and in cell culture experiments we showed that flock scaffolds allow attachment and proliferation of human mesenchymal stem cells and support their osteogenic differentiation. These matrices represent promising scaffolds for tissue engineering.
Keywords: bone; cartilage; flock scaffold; flock technology; tissue engineering.
Figures


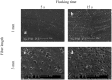

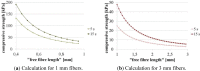
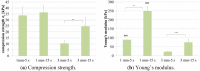
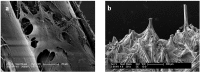


Similar articles
-
Electrostatic flocking of chitosan fibres leads to highly porous, elastic and fully biodegradable anisotropic scaffolds.Acta Biomater. 2016 Oct 15;44:267-76. doi: 10.1016/j.actbio.2016.08.022. Epub 2016 Aug 17. Acta Biomater. 2016. PMID: 27544815
-
Enhanced biochemical and biomechanical properties of scaffolds generated by flock technology for cartilage tissue engineering.Tissue Eng Part A. 2010 Dec;16(12):3697-707. doi: 10.1089/ten.TEA.2009.0817. Epub 2010 Sep 1. Tissue Eng Part A. 2010. PMID: 20673020
-
Electrostatic flocking of salt-treated microfibers and nanofiber yarns for regenerative engineering.Mater Today Bio. 2021 Nov 26;12:100166. doi: 10.1016/j.mtbio.2021.100166. eCollection 2021 Sep. Mater Today Bio. 2021. PMID: 34901819 Free PMC article.
-
Application of textile technology in tissue engineering: A review.Acta Biomater. 2021 Jul 1;128:60-76. doi: 10.1016/j.actbio.2021.04.047. Epub 2021 May 5. Acta Biomater. 2021. PMID: 33962070 Review.
-
Porous Scaffolds for Regeneration of Cartilage, Bone and Osteochondral Tissue.Adv Exp Med Biol. 2018;1058:171-191. doi: 10.1007/978-3-319-76711-6_8. Adv Exp Med Biol. 2018. PMID: 29691822 Review.
Cited by
-
Electrostatic Flocking of Insulative and Biodegradable Polymer Microfibers for Biomedical Applications.Adv Healthc Mater. 2021 Oct;10(19):e2100766. doi: 10.1002/adhm.202100766. Epub 2021 Jul 4. Adv Healthc Mater. 2021. PMID: 34219401 Free PMC article.
-
Bone Tissue Engineering with Adipose-Derived Stem Cells in Bioactive Composites of Laser-Sintered Porous Polycaprolactone Scaffolds and Platelet-Rich Plasma.Materials (Basel). 2013 Oct 25;6(11):4911-4929. doi: 10.3390/ma6114911. Materials (Basel). 2013. PMID: 28788367 Free PMC article.
-
Understanding and utilizing textile-based electrostatic flocking for biomedical applications.Appl Phys Rev. 2021 Dec;8(4):041326. doi: 10.1063/5.0070658. Appl Phys Rev. 2021. PMID: 35003482 Free PMC article. Review.
-
Improved cellular infiltration in electrospun fiber via engineered porosity.Tissue Eng. 2007 Sep;13(9):2249-57. doi: 10.1089/ten.2006.0306. Tissue Eng. 2007. PMID: 17536926 Free PMC article.
-
In Vivo Osteogenic Differentiation of Human Embryoid Bodies in an Injectable in Situ-Forming Hydrogel.Materials (Basel). 2013 Jul 17;6(7):2978-2988. doi: 10.3390/ma6072978. Materials (Basel). 2013. PMID: 28811417 Free PMC article.
References
-
- Sell S.A., Wolfe P.S., Garg K., McCool J.M., Rodriguez I.A., Bowlin G.L. The use of natural polymers in tissue engineering: A focus on electrospun extracellular matrix analogues. Polymers. 2010;2:522–553. doi: 10.3390/polym2040522. - DOI
-
- Houis S., Deichmann T., Veit D., Gries T. Medizinische textilien. In: Wintermantel E., Ha S.W., editors. Medizintechnik: Life Science Engineering. 5th ed. Springer; Berlin, Germany: 2009. pp. 961–992.
-
- Ramakrishna S. Textile scaffolds for tissue engineering. In: Tao X., editor. Smart Fibers, Fabrics and Clothing. CRC Press; Boca Raton, FL, USA: 2001. pp. 291–313.
LinkOut - more resources
Full Text Sources

