Glucose-stimulated insulin release: Parallel perifusion studies of free and hydrogel encapsulated human pancreatic islets
- PMID: 28865118
- PMCID: PMC5699962
- DOI: 10.1002/bit.26442
Glucose-stimulated insulin release: Parallel perifusion studies of free and hydrogel encapsulated human pancreatic islets
Abstract
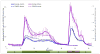
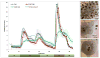
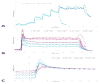
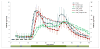
To explore the effects immune-isolating encapsulation has on the insulin secretion of pancreatic islets and to improve our ability to quantitatively describe the glucose-stimulated insulin release (GSIR) of pancreatic islets, we conducted dynamic perifusion experiments with isolated human islets. Free (unencapsulated) and hydrogel encapsulated islets were perifused, in parallel, using an automated multi-channel system that allows sample collection with high temporal resolution. Results indicated that free human islets secrete less insulin per unit mass or islet equivalent (IEQ) than murine islets and with a less pronounced first-phase peak. While small microcapsules (d = 700 µm) caused only a slightly delayed and blunted first-phase insulin response compared to unencapsulated islets, larger capsules (d = 1,800 µm) completely blunted the first-phase peak and decreased the total amount of insulin released. Experimentally obtained insulin time-profiles were fitted with our complex insulin secretion computational model. This allowed further fine-tuning of the hormone-release parameters of this model, which was implemented in COMSOL Multiphysics to couple hormone secretion and nutrient consumption kinetics with diffusive and convective transport. The results of these GSIR experiments, which were also supported by computational modeling, indicate that larger capsules unavoidably lead to dampening of the first-phase insulin response and to a sustained-release type insulin secretion that can only slowly respond to changes in glucose concentration. Bioartificial pancreas type devices can provide long-term and physiologically desirable solutions only if immunoisolation and biocompatibility considerations are integrated with optimized nutrient diffusion and insulin release characteristics by design.
Keywords: FEM model; alginate; diabetes mellitus; fluid dynamics; glucose-stimulated insulin secretion; islet encapsulation.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
The author(s) declare that they have no competing interests.
Figures




Similar articles
-
Experimental evaluation and computational modeling of the effects of encapsulation on the time-profile of glucose-stimulated insulin release of pancreatic islets.Biomed Eng Online. 2015 Mar 28;14:28. doi: 10.1186/s12938-015-0021-9. Biomed Eng Online. 2015. PMID: 25889474 Free PMC article.
-
A local glucose-and oxygen concentration-based insulin secretion model for pancreatic islets.Theor Biol Med Model. 2011 Jun 21;8:20. doi: 10.1186/1742-4682-8-20. Theor Biol Med Model. 2011. PMID: 21693022 Free PMC article.
-
In Vitro Platform for Studying Human Insulin Release Dynamics of Single Pancreatic Islet Microtissues at High Resolution.Adv Biosyst. 2020 Mar;4(3):e1900291. doi: 10.1002/adbi.201900291. Epub 2020 Jan 29. Adv Biosyst. 2020. PMID: 32293140 Free PMC article.
-
Macroencapsulation of rat islets without alteration of insulin secretion kinetics.Exp Clin Endocrinol Diabetes. 2001;109(2):116-9. doi: 10.1055/s-2001-14828. Exp Clin Endocrinol Diabetes. 2001. PMID: 11341299
-
Design of a bioartificial pancreas(+).J Investig Med. 2010 Oct;58(7):831-7. doi: 10.231/JIM.0b013e3181ed3807. J Investig Med. 2010. PMID: 20683347 Free PMC article.
Cited by
-
A bioinspired scaffold for rapid oxygenation of cell encapsulation systems.Nat Commun. 2021 Oct 6;12(1):5846. doi: 10.1038/s41467-021-26126-w. Nat Commun. 2021. PMID: 34615868 Free PMC article.
-
Organoid microphysiological system preserves pancreatic islet function within 3D matrix.Sci Adv. 2021 Feb 12;7(7):eaba5515. doi: 10.1126/sciadv.aba5515. Print 2021 Feb. Sci Adv. 2021. PMID: 33579705 Free PMC article.
-
Decellularized human pancreatic extracellular matrix-based physiomimetic microenvironment for human islet culture.Acta Biomater. 2023 Nov;171:261-272. doi: 10.1016/j.actbio.2023.09.034. Epub 2023 Sep 22. Acta Biomater. 2023. PMID: 37742726 Free PMC article.
-
Transplantation of Macroencapsulated Insulin-Producing Cells.Curr Diab Rep. 2018 Jun 16;18(8):50. doi: 10.1007/s11892-018-1028-y. Curr Diab Rep. 2018. PMID: 29909496 Review.
-
Lotus-root-shaped cell-encapsulated construct as a retrieval graft for long-term transplantation of human iPSC-derived β-cells.iScience. 2021 Apr 1;24(4):102309. doi: 10.1016/j.isci.2021.102309. eCollection 2021 Apr 23. iScience. 2021. PMID: 33997668 Free PMC article.
References
-
- Barrientos R, Baltrusch S, Sigrist S, Legeay G, Belcourt A, Lenzen S. Kinetics of insulin secretion from MIN6 pseudoislets after encapsulation in a prototype device of a bioartificial pancreas. Horm Metab Res. 2009;41(1):5–9. - PubMed
-
- Bloch K, Papismedov E, Yavriyants K, Vorobeychik M, Beer S, Vardi P. Photosynthetic oxygen generator for bioartificial pancreas. Tissue Eng. 2006;12(2):337–344. - PubMed
-
- Bocca N, Pileggi A, Molano RD, Marzorati S, Wu W, Bodor N, et al. Soft corticosteroids for local immunosuppression: exploring the possibility for the use of loteprednol etabonate in islet transplantation. Pharmazie. 2008;63(3):226–232. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

