Emerging roles of linker histones in regulating chromatin structure and function
- PMID: 29018282
- PMCID: PMC5897046
- DOI: 10.1038/nrm.2017.94
Emerging roles of linker histones in regulating chromatin structure and function
Abstract
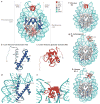
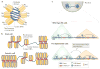
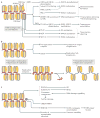
Together with core histones, which make up the nucleosome, the linker histone (H1) is one of the five main histone protein families present in chromatin in eukaryotic cells. H1 binds to the nucleosome to form the next structural unit of metazoan chromatin, the chromatosome, which may help chromatin to fold into higher-order structures. Despite their important roles in regulating the structure and function of chromatin, linker histones have not been studied as extensively as core histones. Nevertheless, substantial progress has been made recently. The first near-atomic resolution crystal structure of a chromatosome core particle and an 11 Å resolution cryo-electron microscopy-derived structure of the 30 nm nucleosome array have been determined, revealing unprecedented details about how linker histones interact with the nucleosome and organize higher-order chromatin structures. Moreover, several new functions of linker histones have been discovered, including their roles in epigenetic regulation and the regulation of DNA replication, DNA repair and genome stability. Studies of the molecular mechanisms of H1 action in these processes suggest a new paradigm for linker histone function beyond its architectural roles in chromatin.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
The Dynamic Influence of Linker Histone Saturation within the Poly-Nucleosome Array.J Mol Biol. 2021 May 14;433(10):166902. doi: 10.1016/j.jmb.2021.166902. Epub 2021 Mar 2. J Mol Biol. 2021. PMID: 33667509 Free PMC article.
-
Chromatin structures condensed by linker histones.Essays Biochem. 2019 Apr 23;63(1):75-87. doi: 10.1042/EBC20180056. Print 2019 Apr 23. Essays Biochem. 2019. PMID: 31015384 Review.
-
Structure and Dynamics of a 197 bp Nucleosome in Complex with Linker Histone H1.Mol Cell. 2017 May 4;66(3):384-397.e8. doi: 10.1016/j.molcel.2017.04.012. Mol Cell. 2017. PMID: 28475873 Free PMC article.
-
Linker histone defines structure and self-association behaviour of the 177 bp human chromatosome.Sci Rep. 2021 Jan 11;11(1):380. doi: 10.1038/s41598-020-79654-8. Sci Rep. 2021. PMID: 33432055 Free PMC article.
-
Linker histones: novel insights into structure-specific recognition of the nucleosome.Biochem Cell Biol. 2017 Apr;95(2):171-178. doi: 10.1139/bcb-2016-0097. Epub 2016 Jun 29. Biochem Cell Biol. 2017. PMID: 28177778 Free PMC article. Review.
Cited by
-
The Dynamic Influence of Linker Histone Saturation within the Poly-Nucleosome Array.J Mol Biol. 2021 May 14;433(10):166902. doi: 10.1016/j.jmb.2021.166902. Epub 2021 Mar 2. J Mol Biol. 2021. PMID: 33667509 Free PMC article.
-
Histone Purification Combined with High-Resolution Mass Spectrometry to Examine Histone Post-Translational Modifications and Histone Variants in Caenorhabditis elegans.Curr Protoc Protein Sci. 2020 Dec;102(1):e114. doi: 10.1002/cpps.114. Curr Protoc Protein Sci. 2020. PMID: 32997895 Free PMC article.
-
Sperm-specific histone H1 in highly condensed sperm nucleus of Sargassum horneri.Sci Rep. 2024 Feb 9;14(1):3387. doi: 10.1038/s41598-024-53729-2. Sci Rep. 2024. PMID: 38336896 Free PMC article.
-
The roles of histone variants in fine-tuning chromatin organization and function.Nat Rev Mol Cell Biol. 2020 Sep;21(9):522-541. doi: 10.1038/s41580-020-0262-8. Epub 2020 Jul 14. Nat Rev Mol Cell Biol. 2020. PMID: 32665685 Free PMC article. Review.
-
Single-stranded nucleic acid binding and coacervation by linker histone H1.Nat Struct Mol Biol. 2022 May;29(5):463-471. doi: 10.1038/s41594-022-00760-4. Epub 2022 Apr 28. Nat Struct Mol Biol. 2022. PMID: 35484234 Free PMC article.
References
-
- Olins AL, Olins DE. Spheroid chromatin units (v bodies) Science. 1974;183:330–332. - PubMed
-
- Kornberg RD. Chromatin structure: a repeating unit of histones and DNA. Science. 1974;184:868–871. - PubMed
-
- Kornberg RD, Thomas JO. Chromatin structure; oligomers of the histones. Science. 1974;184:865–868. - PubMed
-
- Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997;389:251–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

