Low PTEN levels and PIK3CA mutations predict resistance to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2 over-expressing breast cancer
- PMID: 29110152
- PMCID: PMC5821069
- DOI: 10.1007/s10549-017-4533-9
Low PTEN levels and PIK3CA mutations predict resistance to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2 over-expressing breast cancer
Abstract
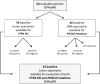
Purpose: Aberrant activation of the PI3K pathway has been implicated in resistance to HER2-_targeted therapy, but results of clinical trials are confounded by the co-administration of chemotherapy. We investigated the effect of perturbations of this pathway in breast cancers from patients treated with neoadjuvant anti-HER2-_targeted therapy without chemotherapy.
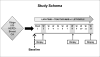
Patients and methods: Baseline tumor samples from patients with HER2-positive breast cancer enrolled in TBCRC006 (NCT00548184), a 12-week neoadjuvant clinical trial with lapatinib plus trastuzumab [plus endocrine therapy for estrogen receptor (ER)-positive tumors], were assessed for PTEN status by immunohistochemistry and PIK3CA mutations by sequencing. Results were correlated with pathologic complete response (pCR).
Results: Of 64 evaluable patients, PTEN immunohistochemistry and PIK3CA mutation analysis were performed for 59 and 46 patients, respectively. PTEN status (dichotomized by H-score median) was correlated with pCR (32% in high PTEN vs. 9% in low PTEN, p = 0.04). PIK3CA mutations were identified in 14/46 tumors at baseline (30%) and did not correlate with ER or PTEN status. One patient whose tumor harbored a PIK3CA mutation achieved pCR (p = 0.14). When considered together (43 cases), 1/25 cases (4%) with a PIK3CA mutation and/or low PTEN expression levels had a pCR compared to 7/18 cases (39%) with wild-type PI3KCA and high PTEN expression levels (p = 0.006).
Conclusion: PI3K pathway activation is associated with resistance to lapatinib and trastuzumab in breast cancers, without chemotherapy. Further studies are warranted to investigate how to use these biomarkers to identify upfront patients who may respond to anti-HER2 alone, without chemotherapy.
Keywords: HER2-positive breast cancer; Lapatinib; PIK3CA mutations; PTEN levels; Trastuzumab; pCR.
Conflict of interest statement
Mothaffar F. Rimawi: Research grant from GlaxoSmithKline (to Institution), Consulting with Genentech.
Carmine De Angelis: Nothing to disclose
Alejandro Contreras: Nothing to disclose
Fresia Pareja: Nothing to disclose
Felipe C. Geyer: Nothing to disclose
Kathleen A. Burke: Nothing to disclose
Sabrina Herrera: Nothing to disclose
Tao Wang: Nothing to disclose
Ingrid A Mayer: Nothing to disclose
Andres Forero: Research grants from GlaxoSmithKline and Genentech (to Institution)
Rita Nanda: Nothing to disclose
Matthew P. Goetz: Nothing to disclose
Jenny C. Chang: Nothing to disclose
Ian E. Krop: Consulting: Genentech/Roche. Research grant from Genentech/Roche (to Institution)
Antonio C. Wolff: Research grant from Genentech (to Institution)
Anne C. Pavlick: Nothing to disclose
Suzanne A. W. Fuqua: Nothing to disclose
Carolina Gutierrez: Nothing to disclose
Susan G. Hilsenbeck: Nothing to disclose
Marilyn M. Li: Nothing to disclose
Britta Weigelt: Nothing to disclose
Jorge S. Reis-Filho: Nothing to disclose
C. Kent Osborne: Nothing to disclose
Rachel Schiff: Nothing to disclose
Figures

Similar articles
-
A combinatorial biomarker predicts pathologic complete response to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2+ breast cancer.Ann Oncol. 2019 Jun 1;30(6):927-933. doi: 10.1093/annonc/mdz076. Ann Oncol. 2019. PMID: 30903140 Free PMC article.
-
Impact of somatic PI3K pathway and ERBB family mutations on pathological complete response (pCR) in HER2-positive breast cancer patients who received neoadjuvant HER2-_targeted therapies.Breast Cancer Res. 2017 Jul 27;19(1):87. doi: 10.1186/s13058-017-0883-9. Breast Cancer Res. 2017. PMID: 28750640 Free PMC article. Clinical Trial.
-
Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers.J Clin Oncol. 2011 Jan 10;29(2):166-73. doi: 10.1200/JCO.2009.27.7814. Epub 2010 Dec 6. J Clin Oncol. 2011. PMID: 21135276 Free PMC article.
-
Tumor-infiltrating lymphocytes in patients with HER2-positive breast cancer treated with neoadjuvant chemotherapy plus trastuzumab, lapatinib or their combination: A meta-analysis of randomized controlled trials.Cancer Treat Rev. 2017 Jun;57:8-15. doi: 10.1016/j.ctrv.2017.04.005. Epub 2017 May 2. Cancer Treat Rev. 2017. PMID: 28525810 Review.
-
A systematic review of dual _targeting in HER2-positive breast cancer.Cancer Treat Rev. 2014 Mar;40(2):259-70. doi: 10.1016/j.ctrv.2013.09.002. Epub 2013 Sep 11. Cancer Treat Rev. 2014. PMID: 24080156 Review.
Cited by
-
Pyrotinib Treatment in Patients With HER2-positive Metastatic Breast Cancer and Brain Metastasis: Exploratory Final Analysis of Real-World, Multicenter Data.Clin Cancer Res. 2021 Aug 15;27(16):4634-4641. doi: 10.1158/1078-0432.CCR-21-0474. Epub 2021 Jun 10. Clin Cancer Res. 2021. PMID: 34112711 Free PMC article.
-
DE-ESCALATING TREATMENT FOR HER2-POSITIVE EARLY BREAST CANCER.Trans Am Clin Climatol Assoc. 2020;131:119-126. Trans Am Clin Climatol Assoc. 2020. PMID: 32675852 Free PMC article.
-
A randomized, controlled phase II trial of neoadjuvant ado-trastuzumab emtansine, lapatinib, and nab-paclitaxel versus trastuzumab, pertuzumab, and paclitaxel in HER2-positive breast cancer (TEAL study).Breast Cancer Res. 2019 Sep 2;21(1):100. doi: 10.1186/s13058-019-1186-0. Breast Cancer Res. 2019. PMID: 31477168 Free PMC article. Clinical Trial.
-
The impact of PI3K inhibitors on breast cancer cell and its tumor microenvironment.PeerJ. 2018 Jun 19;6:e5092. doi: 10.7717/peerj.5092. eCollection 2018. PeerJ. 2018. PMID: 29942710 Free PMC article.
-
A combinatorial biomarker predicts pathologic complete response to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2+ breast cancer.Ann Oncol. 2019 Jun 1;30(6):927-933. doi: 10.1093/annonc/mdz076. Ann Oncol. 2019. PMID: 30903140 Free PMC article.
References
-
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–12. - PubMed
-
- Thor AD, Berry DA, Budman DR, Muss HB, Kute T, Henderson IC, et al. erbB-2, p53, and efficacy of adjuvant therapy in lymph node-positive breast cancer. J Natl Cancer Inst. 1998;90(18):1346–60. - PubMed
-
- Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7(7):505–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

