The role of βII spectrin in cardiac health and disease
- PMID: 29128512
- PMCID: PMC5743760
- DOI: 10.1016/j.lfs.2017.11.009
The role of βII spectrin in cardiac health and disease
Abstract
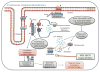
Spectrins are large, flexible proteins comprised of α-β dimers that are connected head-to-head to form the canonical heterotetrameric spectrin structure. Spectrins were initially believed to be exclusively found in human erythrocytic membrane and are highly conserved among different species. βII spectrin, the most common isoform of non-erythrocytic spectrin, is found in all nucleated cells and forms larger macromolecular complexes with ankyrins and actins. Not only is βII spectrin a central cytoskeletal scaffolding protein involved in preserving cell structure but it has also emerged as a critical protein required for distinct physiologic functions such as posttranslational localization of crucial membrane proteins and signal transduction. In the heart, βII spectrin plays a vital role in maintaining normal cardiac membrane excitability and proper cardiac development during embryogenesis. Mutations in βII spectrin genes have been strongly linked with the development of serious cardiac disorders such as congenital arrhythmias, heart failure, and possibly sudden cardiac death. This review focuses on our current knowledge of the role βII spectrin plays in the cardiovascular system in health and disease and the potential future clinical implications.
Keywords: Ankyrin; Cytoskeleton; Spectrin; βII spectrin.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

Similar articles
-
βII spectrin (SPTBN1): biological function and clinical potential in cancer and other diseases.Int J Biol Sci. 2021 Jan 1;17(1):32-49. doi: 10.7150/ijbs.52375. eCollection 2021. Int J Biol Sci. 2021. PMID: 33390831 Free PMC article. Review.
-
Dysfunction in the βII spectrin-dependent cytoskeleton underlies human arrhythmia.Circulation. 2015 Feb 24;131(8):695-708. doi: 10.1161/CIRCULATIONAHA.114.013708. Epub 2015 Jan 28. Circulation. 2015. PMID: 25632041 Free PMC article.
-
Cardiomyocyte βII spectrin plays a critical role in maintaining cardiac function by regulating mitochondrial respiratory function.Cardiovasc Res. 2024 Sep 21;120(11):1312-1326. doi: 10.1093/cvr/cvae116. Cardiovasc Res. 2024. PMID: 38832923
-
Ankyrin-G palmitoylation and βII-spectrin binding to phosphoinositide lipids drive lateral membrane assembly.J Cell Biol. 2014 Jul 21;206(2):273-88. doi: 10.1083/jcb.201401016. J Cell Biol. 2014. PMID: 25049274 Free PMC article.
-
Evolution of spectrin function in cytoskeletal and membrane networks.Biochem Soc Trans. 2009 Aug;37(Pt 4):796-803. doi: 10.1042/BST0370796. Biochem Soc Trans. 2009. PMID: 19614597 Review.
Cited by
-
SPTBN1 Mediates the Cytoplasmic Constraint of PTTG1, Impairing Its Oncogenic Activity in Human Seminoma.Int J Mol Sci. 2023 Nov 29;24(23):16891. doi: 10.3390/ijms242316891. Int J Mol Sci. 2023. PMID: 38069214 Free PMC article.
-
Serum Raman spectroscopy: Unearthing the snapshot of distinct metabolic profile in patients with congenital heart defects (CHDs).Heliyon. 2024 Jul 14;10(16):e34575. doi: 10.1016/j.heliyon.2024.e34575. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39262980 Free PMC article.
-
βII spectrin (SPTBN1): biological function and clinical potential in cancer and other diseases.Int J Biol Sci. 2021 Jan 1;17(1):32-49. doi: 10.7150/ijbs.52375. eCollection 2021. Int J Biol Sci. 2021. PMID: 33390831 Free PMC article. Review.
-
The Spectrinome: The Interactome of a Scaffold Protein Creating Nuclear and Cytoplasmic Connectivity and Function.Exp Biol Med (Maywood). 2019 Nov;244(15):1273-1302. doi: 10.1177/1535370219867269. Epub 2019 Sep 4. Exp Biol Med (Maywood). 2019. PMID: 31483159 Free PMC article. Review.
-
miR-153-3p _targets βII Spectrin to Regulate Formaldehyde-Induced Cardiomyocyte Apoptosis.Front Cardiovasc Med. 2021 Dec 15;8:764831. doi: 10.3389/fcvm.2021.764831. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34977182 Free PMC article.
References
-
- Marchesi VT, Steers E., Jr Selective solubilization of a protein component of the red cell membrane. Science. 1968;159:203–204. http://dx.doi.org/10.1126/science.159.3811.203. - DOI - PubMed
-
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. - PubMed
-
- Hiller G, Weber K. Spectrin is absent in various tissue culture cells. Nature. 1977;266:181–183. http://dx.doi.org/10.1038/266181a0. - DOI - PubMed
-
- Painter RG, Sheetz M, Singer SJ. Detection and ultrastructural localization of human smooth muscle myosin-like molecules in human non-muscle cells by specific antibodies. Proc Natl Acad Sci U S A. 1975;72:1359–1363. http://dx.doi.org/10.1073/pnas.72.4.1359. - DOI - PMC - PubMed
-
- Levine J, Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 1981;90:631–642. http://dx.doi.org/10.1083/jcb.90.3.631. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

