Chemical probes and inhibitors of bromodomains outside the BET family
- PMID: 29170712
- PMCID: PMC5644722
- DOI: 10.1039/c6md00373g
Chemical probes and inhibitors of bromodomains outside the BET family
Abstract
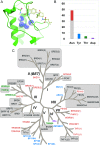
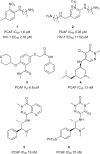
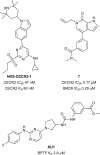
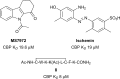
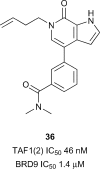
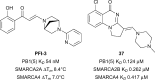
In the last five years, the development of inhibitors of bromodomains has emerged as an area of intensive worldwide research. Emerging evidence has implicated a number of non-BET bromodomains in the onset and progression of diseases such as cancer, HIV infection and inflammation. The development and use of small molecule chemical probes has been fundamental to pre-clinical evaluation of bromodomains as _targets. Recent efforts are described highlighting the development of potent, selective and cell active non-BET bromodomain inhibitors and their therapeutic potential. Over half of typical bromodomains now have reported ligands, but those with atypical binding site residues remain resistant to chemical probe discovery efforts.
Figures
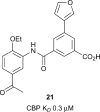
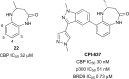
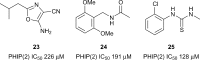
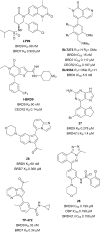





Similar articles
-
Progress in the Development of non-BET Bromodomain Chemical Probes.ChemMedChem. 2016 Mar 4;11(5):477-87. doi: 10.1002/cmdc.201500540. Epub 2016 Jan 8. ChemMedChem. 2016. PMID: 26749027 Review.
-
Advancements in the Development of non-BET Bromodomain Chemical Probes.ChemMedChem. 2019 Feb 19;14(4):362-385. doi: 10.1002/cmdc.201800738. Epub 2019 Feb 4. ChemMedChem. 2019. PMID: 30624862 Review.
-
Bromodomains: Structure, function and pharmacology of inhibition.Biochem Pharmacol. 2016 Apr 15;106:1-18. doi: 10.1016/j.bcp.2015.12.005. Epub 2015 Dec 18. Biochem Pharmacol. 2016. PMID: 26707800 Review.
-
Clinical progress and pharmacology of small molecule bromodomain inhibitors.Curr Opin Chem Biol. 2016 Aug;33:58-66. doi: 10.1016/j.cbpa.2016.05.028. Epub 2016 Jun 10. Curr Opin Chem Biol. 2016. PMID: 27295577 Review.
-
GNE-064: A Potent, Selective, and Orally Bioavailable Chemical Probe for the Bromodomains of SMARCA2 and SMARCA4 and the Fifth Bromodomain of PBRM1.J Med Chem. 2022 Aug 25;65(16):11177-11186. doi: 10.1021/acs.jmedchem.2c00662. Epub 2022 Aug 5. J Med Chem. 2022. PMID: 35930799
Cited by
-
Identification of Selective BRD9 Inhibitor via Integrated Computational Approach.Int J Mol Sci. 2022 Nov 4;23(21):13513. doi: 10.3390/ijms232113513. Int J Mol Sci. 2022. PMID: 36362300 Free PMC article.
-
Modulating the masters: chemical tools to dissect CBP and p300 function.Curr Opin Chem Biol. 2018 Aug;45:195-203. doi: 10.1016/j.cbpa.2018.06.005. Epub 2018 Jul 17. Curr Opin Chem Biol. 2018. PMID: 30025258 Free PMC article. Review.
-
Discovery of a PCAF Bromodomain Chemical Probe.Angew Chem Int Ed Engl. 2017 Jan 16;56(3):827-831. doi: 10.1002/anie.201610816. Epub 2016 Dec 14. Angew Chem Int Ed Engl. 2017. PMID: 27966810 Free PMC article.
-
Improved methods for _targeting epigenetic reader domains of acetylated and methylated lysine.Curr Opin Chem Biol. 2021 Aug;63:132-144. doi: 10.1016/j.cbpa.2021.03.002. Epub 2021 Apr 11. Curr Opin Chem Biol. 2021. PMID: 33852996 Free PMC article. Review.
-
_targeting bromodomain-containing proteins: research advances of drug discovery.Mol Biomed. 2023 May 5;4(1):13. doi: 10.1186/s43556-023-00127-1. Mol Biomed. 2023. PMID: 37142850 Free PMC article. Review.
References
-
- Arrowsmith C. H., Bountra C., Fish P. V., Lee K., Schapira M. Nat. Rev. Drug Discovery. 2012;11:384–400. - PubMed
-
- Esteller M. N. Engl. J. Med. 2008;358:1148–1159. - PubMed
-
- Jones P. A., Laird P. W. Nat. Genet. 1999;21:163–167. - PubMed
-
- Dawson M. A., Kouzarides T., Huntly B. J. P. N. Engl. J. Med. 2012;367:647–657. - PubMed
-
- Shanmugam M. K., Sethi G. Subcell. Biochem. 2013;61:627–657. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

