Hepatic progenitor cell activation in liver repair
- PMID: 29276644
- PMCID: PMC5739327
- DOI: 10.1016/j.livres.2017.08.002
Hepatic progenitor cell activation in liver repair
Abstract
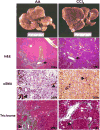
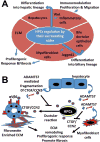
The liver possesses an extraordinary ability to regenerate after injury. Hepatocyte-driven liver regeneration is the default pathway in response to mild-to-moderate acute liver damage. When replication of mature hepatocytes is blocked, facultative hepatic progenitor cells (HPCs), also referred to as oval cells (OCs) in rodents, are activated. HPC/OCs have the ability to proliferate clonogenically and differentiate into several lineages including hepatocytes and bile ductal epithelia. This is a conserved liver injury response that has been studied in many species ranging from mammals (rat, mouse, and human) to fish. In addition, improper HPC/OC activation is closely associated with fibrotic responses, characterized by myofibroblast activation and extracellular matrix production, in many chronic liver diseases. Matrix remodeling and metalloprotease activities play an important role in the regulation of HPC/OC proliferation and fibrosis progression. Thus, understanding molecular mechanisms underlying HPC/OC activation has therapeutic implications for rational design of anti-fibrotic therapies.
Keywords: Hepatic fibrosis; Hepatic progenitor cells (HPCs); Liver injury; Liver regeneration; Oval cells (OCs).
Conflict of interest statement
Conflict of interest The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Significance of CCNs in liver regeneration.J Cell Commun Signal. 2023 Jun;17(2):321-332. doi: 10.1007/s12079-023-00762-x. Epub 2023 May 18. J Cell Commun Signal. 2023. PMID: 37202628 Free PMC article. Review.
-
Blocking development of liver fibrosis augments hepatic progenitor cell-derived liver regeneration in a mouse chronic liver injury model.Hepatol Res. 2019 Sep;49(9):1034-1045. doi: 10.1111/hepr.13351. Epub 2019 May 29. Hepatol Res. 2019. PMID: 30989766
-
Hepatic progenitor cell represents a transitioning cell population between liver epithelium and stroma.Med Hypotheses. 2011 Jun;76(6):809-12. doi: 10.1016/j.mehy.2011.02.024. Epub 2011 Mar 5. Med Hypotheses. 2011. PMID: 21382669
-
Hepatic Progenitor Cells in Action: Liver Regeneration or Fibrosis?Am J Pathol. 2015 Sep;185(9):2342-50. doi: 10.1016/j.ajpath.2015.06.004. Epub 2015 Aug 6. Am J Pathol. 2015. PMID: 26255773 Review.
-
Autophagy promotes hepatic differentiation of hepatic progenitor cells by regulating the Wnt/β-catenin signaling pathway.J Mol Histol. 2019 Feb;50(1):75-90. doi: 10.1007/s10735-018-9808-x. Epub 2019 Jan 2. J Mol Histol. 2019. PMID: 30604254 Free PMC article.
Cited by
-
C3G down-regulation enhances pro-migratory and stemness properties of oval cells by promoting an epithelial-mesenchymal-like process.Int J Biol Sci. 2022 Sep 25;18(15):5873-5884. doi: 10.7150/ijbs.73192. eCollection 2022. Int J Biol Sci. 2022. PMID: 36263169 Free PMC article.
-
Cargo proteins in extracellular vesicles: potential for novel therapeutics in non-alcoholic steatohepatitis.J Nanobiotechnology. 2021 Nov 17;19(1):372. doi: 10.1186/s12951-021-01120-y. J Nanobiotechnology. 2021. PMID: 34789265 Free PMC article.
-
Understanding the marvels behind liver regeneration.Wiley Interdiscip Rev Dev Biol. 2019 May;8(3):e340. doi: 10.1002/wdev.340. Epub 2019 Mar 28. Wiley Interdiscip Rev Dev Biol. 2019. PMID: 30924280 Free PMC article. Review.
-
BMP9 Promotes an Epithelial Phenotype and a Hepatocyte-like Gene Expression Profile in Adult Hepatic Progenitor Cells.Cells. 2022 Jan 21;11(3):365. doi: 10.3390/cells11030365. Cells. 2022. PMID: 35159174 Free PMC article.
-
The combined induction of liver progenitor cells and the suppression of stellate cells by small molecules reverts chronic hepatic dysfunction.Theranostics. 2021 Mar 14;11(11):5539-5552. doi: 10.7150/thno.54457. eCollection 2021. Theranostics. 2021. PMID: 33859762 Free PMC article.
References
-
- Gouw AS, Clouston AD, Theise ND. Ductular reactions in human liver: diversity at the interface. Hepatology. 2011;54:1853–1863. - PubMed
-
- Farber E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956;16:142–148. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
