Tumor response evaluation after neoadjuvant chemotherapy in locally advanced gastric adenocarcinoma: a prospective, multi-center cohort study
- PMID: 29299362
- PMCID: PMC5750190
- DOI: 10.21037/jgo.2017.08.13
Tumor response evaluation after neoadjuvant chemotherapy in locally advanced gastric adenocarcinoma: a prospective, multi-center cohort study
Abstract
Background: To verify the prognostic value of the pathologic and radiological tumor response after neoadjuvant chemotherapy in the treatment of locally advanced gastric adenocarcinoma.
Methods: A total of 67 patients with locally advanced gastric cancer (clinical ≥ T2 or nodal disease and without evidence of distant metastases) underwent perioperative chemotherapy (ECF or ECX regimen) from December 2009 through June 2015 in two surgical units. Histopathological and radiological response to chemotherapy were evaluated by using tumor regression grade (TRG) (Becker's criteria) and volume change assessed by CT.
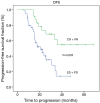
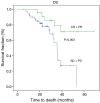
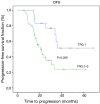
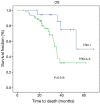
Results: Fifty-one (86%) patients completed all chemotherapy scheduled cycles successfully and surgery was curative (R0) in 64 (97%) subjects. The histopathological analysis showed 19 (29%) specimens with TRG1 (less than 10% of vital tumor left) and 25 (37%) patients had partial or complete response (CR) assessed by CT scan. Median disease free survival (DFS) and overall survival (OS) were 25.70 months (range, 14.52-36.80 months) and 36.60 months (range, 24.3-52.9 months), respectively. The median follow up was 27 months (range, 5.00-68.00 months). Radiological response and TRG were found to be a prognostic factor for OS and DFS, while tumor histology was not significantly related to survival.
Conclusions: Both radiological response and TRG have been shown as promising survival markers in patients treated with perioperative chemotherapy for locally advanced gastric cancer. Other predictive markers of response to chemotherapy are strongly required.
Keywords: Gastric cancer; pathologic response; perioperative chemotherapy; prognostic factors; tumor regression grade (TRG).
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Is pathologic tumor regression grade after neo-adjuvant chemotherapy a promising prognostic indicator for patients with locally advanced gastric cancer? A cohort study evaluating tumor regression response.Cancer Chemother Pharmacol. 2019 Sep;84(3):635-646. doi: 10.1007/s00280-019-03893-4. Epub 2019 Jun 22. Cancer Chemother Pharmacol. 2019. PMID: 31230156
-
Prognostic value of pathological tumor regression grade in locally advanced gastric cancer: New perspectives from a single-center experience.J Surg Oncol. 2021 Mar;123(4):923-931. doi: 10.1002/jso.26391. Epub 2021 Jan 26. J Surg Oncol. 2021. PMID: 33497471 Clinical Trial.
-
College of American Pathologists Tumor Regression Grading System for Long-Term Outcome in Patients with Locally Advanced Rectal Cancer.Oncologist. 2021 May;26(5):e780-e793. doi: 10.1002/onco.13707. Epub 2021 Feb 22. Oncologist. 2021. PMID: 33543577 Free PMC article.
-
[Neoadjuvant chemoradiotherapy combined with surgery versus direct surgery in the treatment of Siewert type II and III adenocarcinomas of the esophagogastric junction: long-term prognostic analysis of a prospective randomized controlled trial].Zhonghua Wei Chang Wai Ke Za Zhi. 2021 Feb 25;24(2):128-137. doi: 10.3760/cma.j.cn.441530-20201019-00565. Zhonghua Wei Chang Wai Ke Za Zhi. 2021. PMID: 33508918 Clinical Trial. Chinese.
-
Predictors and survival for pathologic tumor response grade in borderline resectable and locally advanced pancreatic cancer treated with induction chemotherapy and neoadjuvant stereotactic body radiotherapy.Acta Oncol. 2017 Mar;56(3):391-397. doi: 10.1080/0284186X.2016.1256497. Epub 2016 Nov 25. Acta Oncol. 2017. PMID: 27885876
Cited by
-
MORBIDITY AND SURVIVAL AFTER PERIOPERATIVE CHEMOTHERAPY IN GASTRIC CANCER: A STUDY USING THE BECKER'S CLASSIFICATION AND REGRESSION.Arq Bras Cir Dig. 2023 Jan 9;35:e1704. doi: 10.1590/0102-672020220002e1704. eCollection 2023. Arq Bras Cir Dig. 2023. PMID: 36629685 Free PMC article.
-
Outcomes after Surgical Treatment of Oesophagogastric Cancer with Synchronous Liver Metastases: A Multicentre Retrospective Cohort Study.Cancers (Basel). 2024 Feb 16;16(4):797. doi: 10.3390/cancers16040797. Cancers (Basel). 2024. PMID: 38398190 Free PMC article.
-
Tumor Regression Grade and Overall Survival following Gastrectomy with Preoperative Therapy for Gastric Cancer.Ann Surg Oncol. 2023 Jun;30(6):3580-3589. doi: 10.1245/s10434-023-13151-w. Epub 2023 Feb 10. Ann Surg Oncol. 2023. PMID: 36765008
-
Impact of the Interval between Neoadjuvant Chemotherapy and Gastrectomy on Pathological Response and Survival Outcomes for Patients with Locally Advanced Gastric Cancer: A Meta-analysis.Euroasian J Hepatogastroenterol. 2022 Jul-Dec;12(2):81-91. doi: 10.5005/jp-journals-10018-1382. Euroasian J Hepatogastroenterol. 2022. PMID: 36959991 Free PMC article.
-
Interpretation of the development of neoadjuvant therapy for gastric cancer based on the vicissitudes of the NCCN guidelines.World J Gastrointest Oncol. 2020 Jan 15;12(1):37-53. doi: 10.4251/wjgo.v12.i1.37. World J Gastrointest Oncol. 2020. PMID: 31966912 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
