Anatomical, Physiological, and Functional Diversity of Adipose Tissue
- PMID: 29320711
- PMCID: PMC6050204
- DOI: 10.1016/j.cmet.2017.12.002
Anatomical, Physiological, and Functional Diversity of Adipose Tissue
Abstract
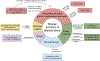
Adipose tissue depots can exist in close association with other organs, where they assume diverse, often non-traditional functions. In stem cell-rich skin, bone marrow, and mammary glands, adipocytes signal to and modulate organ regeneration and remodeling. Skin adipocytes and their progenitors signal to hair follicles, promoting epithelial stem cell quiescence and activation, respectively. Hair follicles signal back to adipocyte progenitors, inducing their expansion and regeneration, as in skin scars. In mammary glands and heart, adipocytes supply lipids to neighboring cells for nutritional and metabolic functions, respectively. Adipose depots adjacent to skeletal structures function to absorb mechanical shock. Adipose tissue near the surface of skin and intestine senses and responds to bacterial invasion, contributing to the body's innate immune barrier. As the recognition of diverse adipose depot functions increases, novel therapeutic approaches centered on tissue-specific adipocytes are likely to emerge for a range of cancers and regenerative, infectious, and autoimmune disorders.
Keywords: adipose stem cells; bone marrow; dermal adipose; hair follicle; mammary gland; mesenteric adipose; regeneration; skin; stem cells; wound healing.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
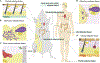


Similar articles
-
Adipocyte differentiation and transdifferentiation: plasticity of the adipose organ.J Endocrinol Invest. 2002 Nov;25(10):823-35. doi: 10.1007/BF03344046. J Endocrinol Invest. 2002. PMID: 12508945 Review.
-
Adipogenic progenitors in different organs: Pathophysiological implications.Rev Endocr Metab Disord. 2022 Feb;23(1):71-85. doi: 10.1007/s11154-021-09686-6. Epub 2021 Oct 29. Rev Endocr Metab Disord. 2022. PMID: 34716543 Free PMC article. Review.
-
Emerging nonmetabolic functions of skin fat.Nat Rev Endocrinol. 2018 Mar;14(3):163-173. doi: 10.1038/nrendo.2017.162. Epub 2018 Jan 12. Nat Rev Endocrinol. 2018. PMID: 29327704 Free PMC article. Review.
-
Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration.Nature. 2008 Jan 17;451(7176):340-4. doi: 10.1038/nature06457. Nature. 2008. PMID: 18202659 Free PMC article.
-
The adipose organ at a glance.Dis Model Mech. 2012 Sep;5(5):588-94. doi: 10.1242/dmm.009662. Dis Model Mech. 2012. PMID: 22915020 Free PMC article. Review.
Cited by
-
Depletion of JunB increases adipocyte thermogenic capacity and ameliorates diet-induced insulin resistance.Metabol Open. 2024 Feb 22;22:100277. doi: 10.1016/j.metop.2024.100277. eCollection 2024 Jun. Metabol Open. 2024. PMID: 39011164 Free PMC article.
-
Of mice and men: Pinpointing species differences in adipose tissue biology.Front Cell Dev Biol. 2022 Sep 15;10:1003118. doi: 10.3389/fcell.2022.1003118. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36187476 Free PMC article. Review.
-
A pig BodyMap transcriptome reveals diverse tissue physiologies and evolutionary dynamics of transcription.Nat Commun. 2021 Jun 17;12(1):3715. doi: 10.1038/s41467-021-23560-8. Nat Commun. 2021. PMID: 34140474 Free PMC article.
-
Diverse repertoire of human adipocyte subtypes develops from transcriptionally distinct mesenchymal progenitor cells.Proc Natl Acad Sci U S A. 2019 Sep 3;116(36):17970-17979. doi: 10.1073/pnas.1906512116. Epub 2019 Aug 16. Proc Natl Acad Sci U S A. 2019. PMID: 31420514 Free PMC article.
-
Emerging Role of Dermal White Adipose Tissue in Modulating Hair Follicle Development During Aging.Front Cell Dev Biol. 2021 Oct 15;9:728188. doi: 10.3389/fcell.2021.728188. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34722509 Free PMC article.
References
-
- Aiache AE, and Ramirez OH (1995). The suborbicularis oculi fat pads: an anatomic and clinical study. Plast Reconstr Surg 95, 37–42. - PubMed
-
- Alexander HG, and Dugdale AE (1992). Fascial planes within subcutaneous fat in humans. European journal of clinical nutrition 46, 903–906. - PubMed
-
- Anderson DB, Kauffman RG, and Kastenschmidt LL (1972). Lipogenic enzyme activities and cellularity of porcine adipose tissue from various anatomical locations. The Journal of Lipid Research 13, 593–599. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

