Sox2 Is Essential for Oligodendroglial Proliferation and Differentiation during Postnatal Brain Myelination and CNS Remyelination
- PMID: 29335358
- PMCID: PMC5815459
- DOI: 10.1523/JNEUROSCI.1291-17.2018
Sox2 Is Essential for Oligodendroglial Proliferation and Differentiation during Postnatal Brain Myelination and CNS Remyelination
Abstract
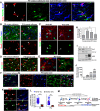
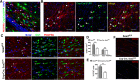
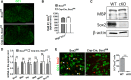
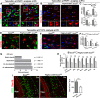
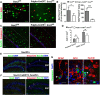
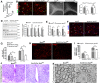
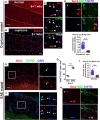
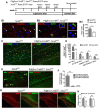
In the CNS, myelination and remyelination depend on the successful progression and maturation of oligodendroglial lineage cells, including proliferation and differentiation of oligodendroglial progenitor cells (OPCs). Previous studies have reported that Sox2 transiently regulates oligodendrocyte (OL) differentiation in the embryonic and perinatal spinal cord and appears dispensable for myelination in the postnatal spinal cord. However, the role of Sox2 in OL development in the brain has yet to be defined. We now report that Sox2 is an essential positive regulator of developmental myelination in the postnatal murine brain of both sexes. Stage-specific paradigms of genetic disruption demonstrated that Sox2 regulated brain myelination by coordinating upstream OPC population supply and downstream OL differentiation. Transcriptomic analyses further supported a crucial role of Sox2 in brain developmental myelination. Consistently, oligodendroglial Sox2-deficient mice developed severe tremors and ataxia, typical phenotypes indicative of hypomyelination, and displayed severe impairment of motor function and prominent deficits of brain OL differentiation and myelination persisting into the later CNS developmental stages. We also found that Sox2 was required for efficient OPC proliferation and expansion and OL regeneration during remyelination in the adult brain and spinal cord. Together, our genetic evidence reveals an essential role of Sox2 in brain myelination and CNS remyelination, and suggests that manipulation of Sox2 and/or Sox2-mediated downstream pathways may be therapeutic in promoting CNS myelin repair.SIGNIFICANCE STATEMENT Promoting myelin formation and repair has translational significance in treating myelin-related neurological disorders, such as periventricular leukomalacia and multiple sclerosis in which brain developmental myelin formation and myelin repair are severely affected, respectively. In this report, analyses of a series of genetic conditional knock-out systems _targeting different oligodendrocyte stages reveal a previously unappreciated role of Sox2 in coordinating upstream proliferation and downstream differentiation of oligodendroglial lineage cells in the mouse brain during developmental myelination and CNS remyelination. Our study points to the potential of manipulating Sox2 and its downstream pathways to promote oligodendrocyte regeneration and CNS myelin repair.
Keywords: Sox2; myelination and remyelination; oligodendrocyte differentiation; oligodendrocyte regeneration; oligodendroglial lineage progression; oligodendroglial progenitor cells.
Copyright © 2018 the authors 0270-6474/18/381802-19$15.00/0.
Figures

Comment in
-
Uncovering the Role of Sox2 in Oligodendroglia.J Neurosci. 2018 May 9;38(19):4460-4461. doi: 10.1523/JNEUROSCI.0556-18.2018. J Neurosci. 2018. PMID: 29743345 Free PMC article. No abstract available.
Similar articles
-
The Stem Cell Factor Sox2 Is a Positive Timer of Oligodendrocyte Development in the Postnatal Murine Spinal Cord.Mol Neurobiol. 2018 Dec;55(12):9001-9015. doi: 10.1007/s12035-018-1035-7. Epub 2018 Apr 5. Mol Neurobiol. 2018. PMID: 29623612 Free PMC article.
-
Loss of Tuberous Sclerosis Complex1 in Adult Oligodendrocyte Progenitor Cells Enhances Axon Remyelination and Increases Myelin Thickness after a Focal Demyelination.J Neurosci. 2017 Aug 2;37(31):7534-7546. doi: 10.1523/JNEUROSCI.3454-16.2017. Epub 2017 Jul 10. J Neurosci. 2017. PMID: 28694334 Free PMC article.
-
HIFα Regulates Developmental Myelination Independent of Autocrine Wnt Signaling.J Neurosci. 2021 Jan 13;41(2):251-268. doi: 10.1523/JNEUROSCI.0731-20.2020. Epub 2020 Nov 18. J Neurosci. 2021. PMID: 33208471 Free PMC article.
-
Engineering biomaterial microenvironments to promote myelination in the central nervous system.Brain Res Bull. 2019 Oct;152:159-174. doi: 10.1016/j.brainresbull.2019.07.013. Epub 2019 Jul 12. Brain Res Bull. 2019. PMID: 31306690 Review.
-
The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination.Adv Exp Med Biol. 1999;468:183-97. doi: 10.1007/978-1-4615-4685-6_15. Adv Exp Med Biol. 1999. PMID: 10635029 Review.
Cited by
-
Developmental thyroid disruption permanently affects the neuroglial output in the murine subventricular zone.Stem Cell Reports. 2022 Mar 8;17(3):459-474. doi: 10.1016/j.stemcr.2022.01.002. Epub 2022 Feb 3. Stem Cell Reports. 2022. PMID: 35120623 Free PMC article.
-
The Stem Cell Factor Sox2 Is a Positive Timer of Oligodendrocyte Development in the Postnatal Murine Spinal Cord.Mol Neurobiol. 2018 Dec;55(12):9001-9015. doi: 10.1007/s12035-018-1035-7. Epub 2018 Apr 5. Mol Neurobiol. 2018. PMID: 29623612 Free PMC article.
-
Hypoxia inducible factor and diffuse white matter injury in the premature brain: perspectives from genetic studies in mice.Neural Regen Res. 2022 Jan;17(1):105-107. doi: 10.4103/1673-5374.314301. Neural Regen Res. 2022. PMID: 34100442 Free PMC article. No abstract available.
-
Transferrin Receptor Is Necessary for Proper Oligodendrocyte Iron Homeostasis and Development.J Neurosci. 2023 May 17;43(20):3614-3629. doi: 10.1523/JNEUROSCI.1383-22.2023. Epub 2023 Mar 28. J Neurosci. 2023. PMID: 36977582 Free PMC article.
-
Using a comprehensive atlas and predictive models to reveal the complexity and evolution of brain-active regulatory elements.Sci Adv. 2024 May 24;10(21):eadj4452. doi: 10.1126/sciadv.adj4452. Epub 2024 May 23. Sci Adv. 2024. PMID: 38781344 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
