Ethnic differences in TGFβ-signaling pathway may contribute to prostate cancer health disparity
- PMID: 29474521
- PMCID: PMC5889036
- DOI: 10.1093/carcin/bgy020
Ethnic differences in TGFβ-signaling pathway may contribute to prostate cancer health disparity
Abstract
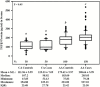
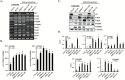
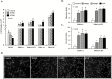
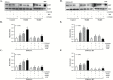
Epidemiological studies show that the incidence and mortality rates of prostate cancer (PCa) are significantly higher in African-American (AA) men when compared with Caucasian (CA) men in the United States. Transforming growth factor β (TGFβ) signaling pathway is linked to health disparities in AAs. Recent studies suggest a role of TGFβ3 in cancer metastases and its effect on the migratory and invasive behavior; however, its role in PCa in AA men has not been studied. We determined the circulating levels of TGFβ3 in AA and CA men diagnosed with PCa using ELISA. We analyzed serum samples from both AA and CA men diagnosed with and without PCa. We show that AA PCa patients had higher levels of TGFβ3 protein compared with AA controls and CA patients. In fact, TGFβ3 protein levels in serum were higher in AA men without PCa compared with the CA population, which may correlate with more aggressive disease seen in AA men. Studies on AA-derived PCa cell lines revealed that TGFβ3 protein levels were also higher in these cells compared with CA-derived PCa cell lines. Our studies also reveal that TGFβ does not inhibit cell proliferation in AA-derived PCa cell lines, but it does induce migration and invasion through activation of PI3K pathway. We suggest that increased TGFβ3 levels are responsible for development of aggressive PCa in AA patients as a consequence of development of resistance to inhibitory effects of TGFβ on cell proliferation and induction of invasive metastatic behavior.
Figures
Similar articles
-
Epigenetic analysis identifies factors driving racial disparity in prostate cancer.Cancer Rep (Hoboken). 2019 Apr;2(2):e1153. doi: 10.1002/cnr2.1153. Epub 2018 Dec 13. Cancer Rep (Hoboken). 2019. PMID: 32721098 Free PMC article.
-
Expression of TGFβ3 and its effects on migratory and invasive behavior of prostate cancer cells: involvement of PI3-kinase/AKT signaling pathway.Clin Exp Metastasis. 2013 Jan;30(1):13-23. doi: 10.1007/s10585-012-9494-0. Epub 2012 Jun 8. Clin Exp Metastasis. 2013. PMID: 22678424 Free PMC article.
-
Racial disparity in prostate cancer in the African American population with actionable ideas and novel immunotherapies.Cancer Rep (Hoboken). 2021 Oct;4(5):e1340. doi: 10.1002/cnr2.1340. Epub 2021 Feb 17. Cancer Rep (Hoboken). 2021. PMID: 33599076 Free PMC article. Review.
-
Differences in the aggressiveness of prostate cancer among Korean, Caucasian, and African American men: A retrospective cohort study of radical prostatectomy.Urol Oncol. 2016 Jan;34(1):3.e9-14. doi: 10.1016/j.urolonc.2015.08.004. Epub 2015 Sep 4. Urol Oncol. 2016. PMID: 26345648
-
Is active surveillance a suitable option for African American men with prostate cancer? A systemic literature review.Prostate Cancer Prostatic Dis. 2017 Jun;20(2):127-136. doi: 10.1038/pcan.2016.56. Epub 2017 Apr 18. Prostate Cancer Prostatic Dis. 2017. PMID: 28417980 Review.
Cited by
-
Race, Gene Expression Signatures, and Clinical Outcomes of Patients With High-Risk Early Breast Cancer.JAMA Netw Open. 2023 Dec 1;6(12):e2349646. doi: 10.1001/jamanetworkopen.2023.49646. JAMA Netw Open. 2023. PMID: 38153734 Free PMC article.
-
Prostate cancer metastasis and health disparities: a systematic review.Prostate Cancer Prostatic Dis. 2024 Jun;27(2):183-191. doi: 10.1038/s41391-023-00667-1. Epub 2023 Apr 12. Prostate Cancer Prostatic Dis. 2024. PMID: 37046071 Review.
-
Biological Basis of Breast Cancer-Related Disparities in Precision Oncology Era.Int J Mol Sci. 2024 Apr 8;25(7):4113. doi: 10.3390/ijms25074113. Int J Mol Sci. 2024. PMID: 38612922 Free PMC article. Review.
-
Racially Disparate Expression of mTOR/ERK-1/2 Allied Proteins in Cancer.Front Cell Dev Biol. 2021 Apr 30;9:601929. doi: 10.3389/fcell.2021.601929. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33996789 Free PMC article. Review.
-
The Relative Importance of Race Compared to Health Care and Social Factors in Predicting Prostate Cancer Mortality: A Random Forest Approach.J Urol. 2019 Dec;202(6):1209-1216. doi: 10.1097/JU.0000000000000416. Epub 2019 Jun 27. J Urol. 2019. PMID: 31246547 Free PMC article.
References
-
- Siegel R.L., et al. (2017)Cancer statistics, 2017. CA. Cancer J. Clin., 67, 7–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

