JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a Phase 2 randomized controlled clinical trial
- PMID: 29481660
- PMCID: PMC6212720
- DOI: 10.1093/ndt/gfx377
JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a Phase 2 randomized controlled clinical trial
Abstract
Background: Inflammation signaled by Janus kinases (JAKs) promotes progression of diabetic kidney disease (DKD). Baricitinib is an oral, reversible, selective inhibitor of JAK1 and JAK2. This study tested the efficacy of baricitinib versus placebo on albuminuria in adults with Type 2 diabetes at high risk for progressive DKD.
Methods: In this Phase 2, double-blind, dose-ranging study, participants were randomized 1:1:1:1:1 to receive placebo or baricitinib (0.75 mg daily; 0.75 mg twice daily; 1.5 mg daily; or 4 mg daily), for 24 weeks followed by 4-8 weeks of washout.
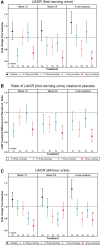
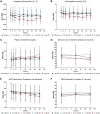
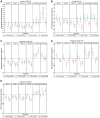
Results: Participants (N = 129) were 63±9.1 (mean±standard deviation) years of age, 27.1% (35/129) women and 11.6% (15/129) African-American race. Baseline hemoglobin A1c (HbA1c) was 7.3±1% and estimated glomerular filtration rate was 45.0±12.1 mL/min/1.73 m2 with first morning urine albumin-creatinine ratio (UACR) of 820 (407-1632) (median; interquartile range) mg/g. Baricitinib, 4 mg daily, decreased morning UACR by 41% at Week 24 compared with placebo (ratio to baseline 0.59, 95% confidence interval 0.38-0.93, P = 0.022). UACR was decreased at Weeks 12 and 24 and after 4-8 weeks of washout. Baricitinib 4 mg decreased inflammatory biomarkers over 24 weeks (urine C-X-C motif chemokine 10 and urine C-C motif ligand 2, plasma soluble tumor necrosis factor receptors 1 and 2, intercellular adhesion molecule 1 and serum amyloid A). The only adverse event rate that differed between groups was anemia at 32.0% (8/25) for baricitinib 4 mg daily versus 3.7% (1/27) for placebo.
Conclusions: Baricitinib decreased albuminuria in participants with Type 2 diabetes and DKD. Further research is required to determine if baricitinib reduces DKD progression.
Figures
Similar articles
-
Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study.Ann Rheum Dis. 2017 Jan;76(1):88-95. doi: 10.1136/annrheumdis-2016-210094. Epub 2016 Sep 29. Ann Rheum Dis. 2017. PMID: 27689735 Free PMC article. Clinical Trial.
-
Baricitinib versus Placebo or Adalimumab in Rheumatoid Arthritis.N Engl J Med. 2017 Feb 16;376(7):652-662. doi: 10.1056/NEJMoa1608345. N Engl J Med. 2017. PMID: 28199814 Clinical Trial.
-
Baricitinib in adult patients with moderate-to-severe atopic dermatitis: A phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study.J Am Acad Dermatol. 2019 Apr;80(4):913-921.e9. doi: 10.1016/j.jaad.2018.01.018. Epub 2018 Feb 2. J Am Acad Dermatol. 2019. PMID: 29410014 Clinical Trial.
-
Baricitinib: A Review in Rheumatoid Arthritis.Drugs. 2018 May;78(7):761-772. doi: 10.1007/s40265-018-0908-4. Drugs. 2018. PMID: 29687421 Review.
-
The effect of JAK1/JAK2 inhibition in rheumatoid arthritis: efficacy and safety of baricitinib.Clin Exp Rheumatol. 2019 Jul-Aug;37(4):694-704. Epub 2019 Feb 11. Clin Exp Rheumatol. 2019. PMID: 30767864 Review.
Cited by
-
Effects of metabolic memory on inflammation and fibrosis associated with diabetic kidney disease: an epigenetic perspective.Clin Epigenetics. 2021 Apr 21;13(1):87. doi: 10.1186/s13148-021-01079-5. Clin Epigenetics. 2021. PMID: 33883002 Free PMC article. Review.
-
Diabetic Kidney Disease Represents a Locus of Opportunity.Front Physiol. 2021 Mar 8;12:650503. doi: 10.3389/fphys.2021.650503. eCollection 2021. Front Physiol. 2021. PMID: 33762972 Free PMC article. No abstract available.
-
Repurposing a clinically approved prescription Colquhounia root tablet to treat diabetic kidney disease via suppressing PI3K/AKT/NF-kB activation.Chin Med. 2022 Jan 4;17(1):2. doi: 10.1186/s13020-021-00563-7. Chin Med. 2022. PMID: 34980163 Free PMC article.
-
Metabolic Inflammation and Insulin Resistance in Obesity.Circ Res. 2020 May 22;126(11):1549-1564. doi: 10.1161/CIRCRESAHA.119.315896. Epub 2020 May 21. Circ Res. 2020. PMID: 32437299 Free PMC article. Review.
-
Signaling Pathways Involved in Diabetic Renal Fibrosis.Front Cell Dev Biol. 2021 Jul 12;9:696542. doi: 10.3389/fcell.2021.696542. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34327204 Free PMC article. Review.
References
-
- ANZDATA Registry. 38th Report: Incidence of End Stage Kidney Disease. Adelaide, Australia: Australia and New Zealand Dialysis and Transplant Registry, 2016
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

