Identification of placental nutrient transporters associated with intrauterine growth restriction and pre-eclampsia
- PMID: 29499643
- PMCID: PMC5833046
- DOI: 10.1186/s12864-018-4518-z
Identification of placental nutrient transporters associated with intrauterine growth restriction and pre-eclampsia
Abstract
Background: Gestational disorders such as intrauterine growth restriction (IUGR) and pre-eclampsia (PE) are main causes of poor perinatal outcomes worldwide. Both diseases are related with impaired materno-fetal nutrient transfer, but the crucial transport mechanisms underlying IUGR and PE are not fully elucidated. In this study, we aimed to identify membrane transporters highly associated with transplacental nutrient deficiencies in IUGR/PE.
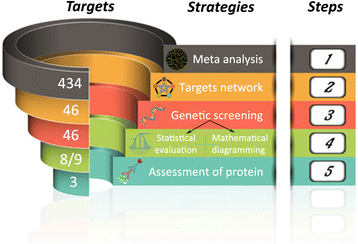
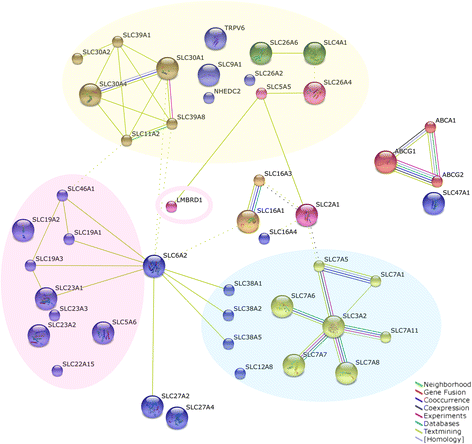
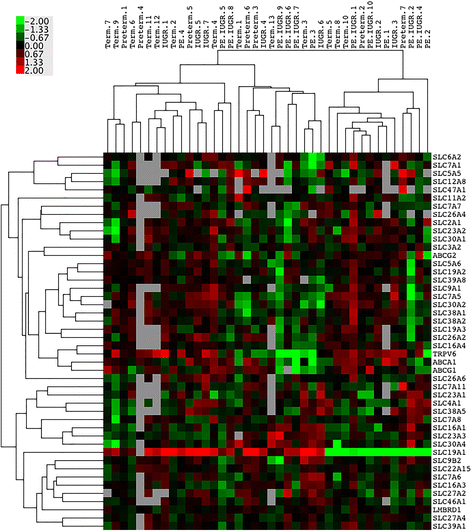
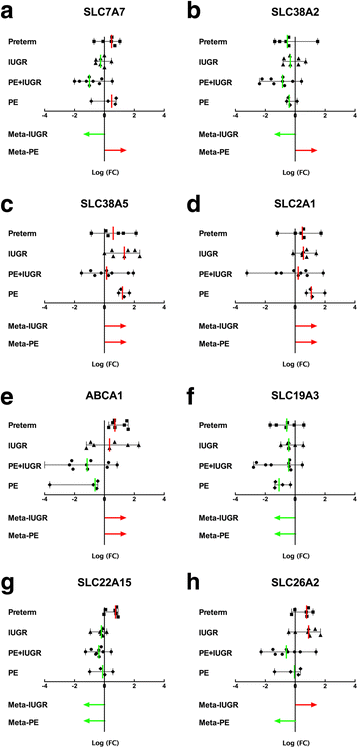
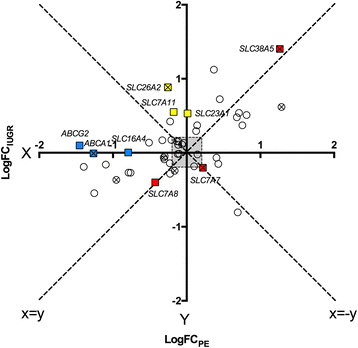
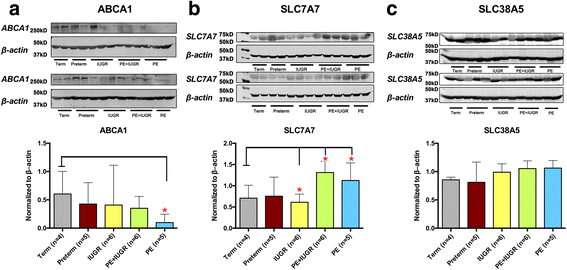
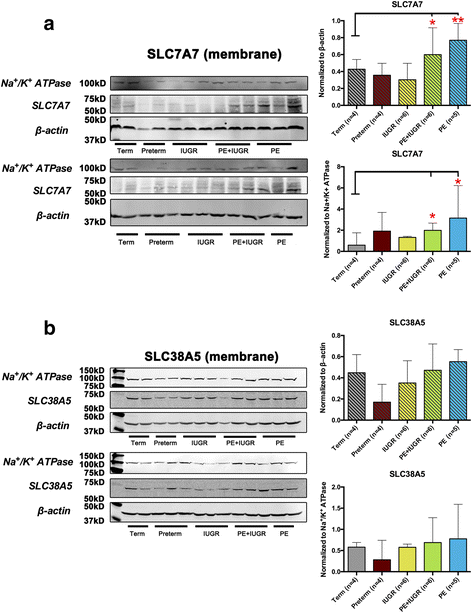
Results: In silico analyses on the identification of differentially expressed nutrient transporters were conducted using seven eligible microarray datasets (from Gene Expression Omnibus), encompassing control and IUGR/PE placental samples. Thereby 46 out of 434 genes were identified as potentially interesting _targets. They are involved in the fetal provision with amino acids, carbohydrates, lipids, vitamins and microelements. _targets of interest were clustered into a substrate-specific interaction network by using Search Tool for the Retrieval of Interacting Genes. The subsequent wet-lab validation was performed using quantitative RT-PCR on placentas from clinically well-characterized IUGR/PE patients (IUGR, n = 8; PE, n = 5; PE+IUGR, n = 10) and controls (term, n = 13; preterm, n = 7), followed by 2D-hierarchical heatmap generation. Statistical evaluation using Kruskal-Wallis tests was then applied to detect significantly different expression patterns, while scatter plot analysis indicated which transporters were predominantly influenced by IUGR or PE, or equally affected by both diseases. Identified by both methods, three overlapping _targets, SLC7A7, SLC38A5 (amino acid transporters), and ABCA1 (cholesterol transporter), were further investigated at the protein level by western blotting. Protein analyses in total placental tissue lysates and membrane fractions isolated from disease and control placentas indicated an altered functional activity of those three nutrient transporters in IUGR/PE.
Conclusions: Combining bioinformatic analysis, molecular biological experiments and mathematical diagramming, this study has demonstrated systematic alterations of nutrient transporter expressions in IUGR/PE. Among 46 initially _targeted transporters, three significantly regulated genes were further investigated based on the severity and the disease specificity for IUGR and PE. Confirmed by mRNA and protein expression, the amino acid transporters SLC7A7 and SLC38A5 showed marked differences between controls and IUGR/PE and were regulated by both diseases. In contrast, ABCA1 may play an exclusive role in the development of PE.
Keywords: Bioinformatics; Intrauterine growth restriction; Membrane transporters; Placenta; Pre-eclampsia.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol was approved by the local ethic committees of the Canton of Bern, Switzerland (178/03). Written informed consent was obtained from each participant for this research prior to the caesarean section. A copy of the consent form is available for review by the Editor of this journal.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Placental ABCA1 and ABCG1 expression in gestational disease: Pre-eclampsia affects ABCA1 levels in syncytiotrophoblasts.Placenta. 2013 Nov;34(11):1079-86. doi: 10.1016/j.placenta.2013.06.309. Epub 2013 Jul 20. Placenta. 2013. PMID: 23880356
-
Differential levels of amino acid transporters System L and ASCT2, and the mTOR protein in placenta of preeclampsia and IUGR.BMC Pregnancy Childbirth. 2014 May 30;14:181. doi: 10.1186/1471-2393-14-181. BMC Pregnancy Childbirth. 2014. PMID: 24886642 Free PMC article.
-
Placental mitochondrial content and function in intrauterine growth restriction and preeclampsia.Am J Physiol Endocrinol Metab. 2014 Feb 15;306(4):E404-13. doi: 10.1152/ajpendo.00426.2013. Epub 2013 Dec 17. Am J Physiol Endocrinol Metab. 2014. PMID: 24347055
-
Epigenetic processes during preeclampsia and effects on fetal development and chronic health.Clin Sci (Lond). 2021 Oct 15;135(19):2307-2327. doi: 10.1042/CS20190070. Clin Sci (Lond). 2021. PMID: 34643675 Free PMC article. Review.
-
The placenta in pre-eclampsia and intrauterine growth restriction.J Clin Pathol. 2008 Dec;61(12):1254-60. doi: 10.1136/jcp.2008.055236. Epub 2008 Jul 19. J Clin Pathol. 2008. PMID: 18641412 Review.
Cited by
-
Regulation of placental amino acid transport in health and disease.Acta Physiol (Oxf). 2024 Jul;240(7):e14157. doi: 10.1111/apha.14157. Epub 2024 May 6. Acta Physiol (Oxf). 2024. PMID: 38711335 Review.
-
Sex-Biased lncRNA Signature in Fetal Growth Restriction (FGR).Cells. 2021 Apr 16;10(4):921. doi: 10.3390/cells10040921. Cells. 2021. PMID: 33923632 Free PMC article.
-
Primary Human Trophoblasts Mimic the Preeclampsia Phenotype after Acute Hypoxia-Reoxygenation Insult.Cells. 2022 Jun 11;11(12):1898. doi: 10.3390/cells11121898. Cells. 2022. PMID: 35741027 Free PMC article.
-
Δ9-tetrahydrocannabinol exposure during rat pregnancy leads to symmetrical fetal growth restriction and labyrinth-specific vascular defects in the placenta.Sci Rep. 2020 Jan 17;10(1):544. doi: 10.1038/s41598-019-57318-6. Sci Rep. 2020. PMID: 31953475 Free PMC article.
-
Development of an Organ-on-a-Chip-Device for Study of Placental Pathologies.Int J Mol Sci. 2020 Nov 19;21(22):8755. doi: 10.3390/ijms21228755. Int J Mol Sci. 2020. PMID: 33228194 Free PMC article.
References
-
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J, Maternal, Child Undernutrition Study G Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–260. - PubMed
-
- Wu G, Imhoff-Kunsch B, Girard AW. Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr Perinat Epidemiol. 2012;26(Suppl 1):4–26. - PubMed
-
- Pathology of the human placenta. http://www.springer.com/de/book/9783642239403
-
- Manual of Pathology of the Human Placenta. https://link.springer.com/book/10.1007%2F978-1-4419-7494-5
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

