Chemotherapeutic-Induced Cardiovascular Dysfunction: Physiological Effects, Early Detection-The Role of Telomerase to Counteract Mitochondrial Defects and Oxidative Stress
- PMID: 29534446
- PMCID: PMC5877658
- DOI: 10.3390/ijms19030797
Chemotherapeutic-Induced Cardiovascular Dysfunction: Physiological Effects, Early Detection-The Role of Telomerase to Counteract Mitochondrial Defects and Oxidative Stress
Abstract
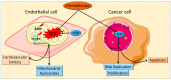
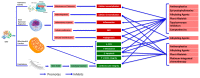
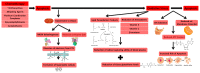
Although chemotherapeutics can be highly effective at _targeting malignancies, their ability to trigger cardiovascular morbidity is clinically significant. Chemotherapy can adversely affect cardiovascular physiology, resulting in the development of cardiomyopathy, heart failure and microvascular defects. Specifically, anthracyclines are known to cause an excessive buildup of free radical species and mitochondrial DNA damage (mtDNA) that can lead to oxidative stress-induced cardiovascular apoptosis. Therefore, oncologists and cardiologists maintain a network of communication when dealing with patients during treatment in order to treat and prevent chemotherapy-induced cardiovascular damage; however, there is a need to discover more accurate biomarkers and therapeutics to combat and predict the onset of cardiovascular side effects. Telomerase, originally discovered to promote cellular proliferation, has recently emerged as a potential mechanism to counteract mitochondrial defects and restore healthy mitochondrial vascular phenotypes. This review details mechanisms currently used to assess cardiovascular damage, such as C-reactive protein (CRP) and troponin levels, while also unearthing recently researched biomarkers, including circulating mtDNA, telomere length and telomerase activity. Further, we explore a potential role of telomerase in the mitigation of mitochondrial reactive oxygen species and maintenance of mtDNA integrity. Telomerase activity presents a promising indicator for the early detection and treatment of chemotherapy-derived cardiac damage.
Keywords: cardiac oncology; heart failure; mtDNA damage; telomerase; telomerase activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Drug-induced mitochondrial dysfunction and cardiotoxicity.Am J Physiol Heart Circ Physiol. 2015 Nov;309(9):H1453-67. doi: 10.1152/ajpheart.00554.2015. Epub 2015 Sep 18. Am J Physiol Heart Circ Physiol. 2015. PMID: 26386112 Free PMC article. Review.
-
Improving the preclinical models for the study of chemotherapy-induced cardiotoxicity: a Position Paper of the Italian Working Group on Drug Cardiotoxicity and Cardioprotection.Heart Fail Rev. 2015 Sep;20(5):621-31. doi: 10.1007/s10741-015-9497-4. Heart Fail Rev. 2015. PMID: 26168714
-
Autophagy and mitophagy in the context of doxorubicin-induced cardiotoxicity.Onco_target. 2017 Jul 11;8(28):46663-46680. doi: 10.18632/onco_target.16944. Onco_target. 2017. PMID: 28445146 Free PMC article. Review.
-
Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress.J Cell Sci. 2008 Apr 1;121(Pt 7):1046-53. doi: 10.1242/jcs.019372. Epub 2008 Mar 11. J Cell Sci. 2008. PMID: 18334557
-
Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage.Arterioscler Thromb Vasc Biol. 2009 Jun;29(6):929-35. doi: 10.1161/ATVBAHA.109.185546. Epub 2009 Mar 5. Arterioscler Thromb Vasc Biol. 2009. PMID: 19265030
Cited by
-
Is There a Mitochondrial Protection via Remote Ischemic Conditioning in Settings of Anticancer Therapy Cardiotoxicity?Curr Heart Fail Rep. 2024 Aug;21(4):292-304. doi: 10.1007/s11897-024-00658-w. Epub 2024 Mar 21. Curr Heart Fail Rep. 2024. PMID: 38512567 Free PMC article. Review.
-
Greenspace, Inflammation, Cardiovascular Health, and Cancer: A Review and Conceptual Framework for Greenspace in Cardio-Oncology Research.Int J Environ Res Public Health. 2022 Feb 19;19(4):2426. doi: 10.3390/ijerph19042426. Int J Environ Res Public Health. 2022. PMID: 35206610 Free PMC article. Review.
-
Melanocortin 1 receptor attenuates early brain injury following subarachnoid hemorrhage by controlling mitochondrial metabolism via AMPK/SIRT1/PGC-1α pathway in rats.Theranostics. 2021 Jan 1;11(2):522-539. doi: 10.7150/thno.49426. eCollection 2021. Theranostics. 2021. PMID: 33391490 Free PMC article.
-
Investigating the association between blood metabolites and telomere length: A mendelian randomization study.PLoS One. 2024 Mar 8;19(3):e0298172. doi: 10.1371/journal.pone.0298172. eCollection 2024. PLoS One. 2024. PMID: 38457472 Free PMC article.
-
Diverse Roles of Mitochondria in Immune Responses: Novel Insights Into Immuno-Metabolism.Front Immunol. 2018 Jul 12;9:1605. doi: 10.3389/fimmu.2018.01605. eCollection 2018. Front Immunol. 2018. PMID: 30050539 Free PMC article. Review.
References
-
- Ai D., Banchs J., Owusu-Agyemang P., Cata J.P. Chemotherapy-induced cardiovascular toxicity: Beyond anthracyclines. Minerva Anestesiol. 2014;80:586–594. - PubMed
-
- Guarini G., Kiyooka T., Ohanyan V., Pung Y.F., Marzilli M., Chen Y.R., Chen C.L., Kang P.T., Hardwick J.P., Kolz C.L., et al. Impaired coronary metabolic dilation in the metabolic syndrome is linked to mitochondrial dysfunction and mitochondrial DNA damage. Basic Res. Cardiol. 2016;111:29. doi: 10.1007/s00395-016-0547-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

