Co-delivery of Aurora-A inhibitor XY-4 and Bcl-xl siRNA enhances antitumor efficacy for melanoma therapy
- PMID: 29563798
- PMCID: PMC5849942
- DOI: 10.2147/IJN.S147759
Co-delivery of Aurora-A inhibitor XY-4 and Bcl-xl siRNA enhances antitumor efficacy for melanoma therapy
Abstract
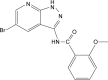
Background: The newly synthesized Aurora-A kinase inhibitor XY-4 is a potential anti-cancer agent, but its hydrophobicity and limited efficiency restrict further application. Nanotechnology based combined therapy provides an optimized strategy for solving these issues.
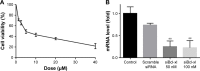
Methods: In this study, the newly synthesized Aurora-A kinase inhibitor XY-4 and Bcl-xl _targeted siRNA were co-delivered by cationic liposomes, creating an injectable co-delivery formulation. The anti-cancer ability and mechanisms of XY-4/Bcl-xl siRNA co-loaded cationic liposomes were studied both in vitro and in vivo.
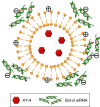
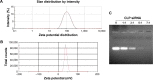
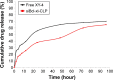
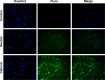
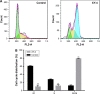
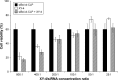
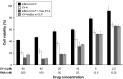
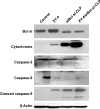
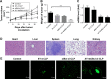
Results: The prepared liposomes had a mean particle size of 91.3±4.5 nm with a zeta potential of 38.5±0.5 mV and were monodispersed (Polydispersity index =0.183) in water solution, with high drug loading capacity and stability. Intriguingly, the positive charges of co-delivery liposomes not only facilitated gene delivery, but also obviously enhanced drug uptake. The XY-4/Bcl-xl siRNA co-loaded cationic liposomes demonstrated enhanced anti-cancer effects on B16 melanoma cells in vitro by activation mitochondrial apoptosis pathway. Moreover, intratumoral injection of this co-delivery formulation efficiently inhibited the growth of a B16 melanoma xenograft model in vivo.
Conclusion: By co-delivering Aurora-A kinase inhibitor XY-4 and Bcl-xl _targeting siRNA in a nanoformulation, our study supplied a potential combination strategy for melanoma therapy.
Keywords: Aurora-A kinase inhibitor; RNA interference; apoptosis; co-delivery; liposome; melanoma.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Improving Skin Cancer Treatment by Dual Drug Co-Encapsulation into Liposomal Systems-An Integrated Approach towards Anticancer Synergism and _targeted Delivery.Pharmaceutics. 2024 Sep 12;16(9):1200. doi: 10.3390/pharmaceutics16091200. Pharmaceutics. 2024. PMID: 39339235 Free PMC article. Review.
-
Systemic delivery of siRNA specific to tumor mediated by atelocollagen: combined therapy using siRNA _targeting Bcl-xL and cisplatin against prostate cancer.Int J Cancer. 2009 Dec 15;125(12):2978-90. doi: 10.1002/ijc.24382. Int J Cancer. 2009. PMID: 19422046
-
Effective Skin Cancer Treatment by Topical Co-delivery of Curcumin and STAT3 siRNA Using Cationic Liposomes.AAPS PharmSciTech. 2018 Jan;19(1):166-175. doi: 10.1208/s12249-017-0833-y. Epub 2017 Jun 21. AAPS PharmSciTech. 2018. PMID: 28639178
-
Therapeutic _targeting of siRNA/anti-cancer drug delivery system for non-melanoma skin cancer. Part I: Development and gene silencing of JAK1siRNA/5-FU loaded liposome nanocomplexes.Eur J Pharm Biopharm. 2024 Oct;203:114432. doi: 10.1016/j.ejpb.2024.114432. Epub 2024 Aug 2. Eur J Pharm Biopharm. 2024. PMID: 39097115
-
Assembly strategy of liposome and polymer systems for siRNA delivery.Int J Pharm. 2021 Jan 5;592:120033. doi: 10.1016/j.ijpharm.2020.120033. Epub 2020 Nov 1. Int J Pharm. 2021. PMID: 33144189 Review.
Cited by
-
Improving Skin Cancer Treatment by Dual Drug Co-Encapsulation into Liposomal Systems-An Integrated Approach towards Anticancer Synergism and _targeted Delivery.Pharmaceutics. 2024 Sep 12;16(9):1200. doi: 10.3390/pharmaceutics16091200. Pharmaceutics. 2024. PMID: 39339235 Free PMC article. Review.
-
Development, Characterization and Use of Liposomes as Amphipathic Transporters of Bioactive Compounds for Melanoma Treatment and Reduction of Skin Inflammation: A Review.Int J Nanomedicine. 2020 Oct 8;15:7627-7650. doi: 10.2147/IJN.S263516. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33116492 Free PMC article. Review.
-
Cationic micelle-based siRNA delivery for efficient colon cancer gene therapy.Nanoscale Res Lett. 2019 Jun 4;14(1):193. doi: 10.1186/s11671-019-2985-z. Nanoscale Res Lett. 2019. PMID: 31165329 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. - PubMed
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. - PubMed
-
- Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new _targeted therapy. Nature. 2007;445(7130):851–857. - PubMed
-
- Lapenna S, Giordano A. Cell cycle kinases as therapeutic _targets for cancer. Nature Rev Drug Discov. 2009;8(7):547–566. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

