Evaluation of combined growth media for in vitro cultivation of oropharyngeal biofilms on prosthetic silicone
- PMID: 29633010
- PMCID: PMC5891558
- DOI: 10.1007/s10856-018-6051-7
Evaluation of combined growth media for in vitro cultivation of oropharyngeal biofilms on prosthetic silicone
Abstract
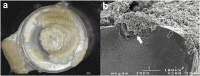
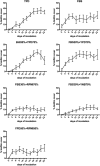
In the upper aerodigestive tract, biofilm deposits by oropharyngeal microbes can cause failure of medical polymer devices like voice prostheses. Previous studies on testing of inhibitive strategies still lack of comparability due to varying study protocols concerning growth media, microbial species and growth conditions. Goal of the study was therefore to test cultivation of a mixed biofilm of isolated oropharyngeal microbes under in vitro growth conditions using mixtures of common growth media. Mixtures of yeast peptone dextrose medium (YPD), fetal bovine serum (FBS), RPMI 1640, Yeast nitrogen base medium (YNB) and brain heart infusion (BHI) were tested to grow mixed biofilm deposits of Candida albicans, Candida tropicalis, Staphylococcus aureus, Streptococcus epidermidis, Rothia dentocariosa and Lactobacillus gasseri on medical grade silicone. Periodic assessment of living biofilm was performed over 22 days by a digital microscope and the cultivated biofilm structures were analyzed by scanning electron microscopy after completion of the study. Mixtures of BHI, YPD and FBS improved microscopic growth of multispecies biofilm deposits over time, while addition of RPMI and YNB resulted in reduction of visible biofilm deposit sizes. A mixtures of FBS 30% + YPD 70% and BHI 30% + YPD 70% showed enhanced support of permanent surface growth on silicone. Growth kinetics of in vitro multispecies biofilms can be manipulated by using mixtures of common growth media. Using mixtures of growth media can improve growth of longterm multispecies oropharyngeal biofilm models used for in vitro testing of antibiofilm materials or coatings for voice prostheses.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Growth Media for Mixed Multispecies Oropharyngeal Biofilm Compositions on Silicone.Biomed Res Int. 2019 Jul 9;2019:8051270. doi: 10.1155/2019/8051270. eCollection 2019. Biomed Res Int. 2019. PMID: 31360725 Free PMC article.
-
Long-term antibiofilm activity of carboxymethyl chitosan on mixed biofilm on silicone.Laryngoscope. 2016 Dec;126(12):E404-E408. doi: 10.1002/lary.26096. Epub 2016 Jun 27. Laryngoscope. 2016. PMID: 27346839
-
Evaluation of culture conditions for mixed biofilm formation with clinically isolated non-albicans Candida species and Staphylococcus epidermidis on silicone.Microb Pathog. 2017 Nov;112:215-220. doi: 10.1016/j.micpath.2017.10.002. Epub 2017 Oct 5. Microb Pathog. 2017. PMID: 28987620
-
Candida biofilm formation on voice prostheses.J Med Microbiol. 2015 Mar;64(Pt 3):199-208. doi: 10.1099/jmm.0.078717-0. Epub 2014 Aug 8. J Med Microbiol. 2015. PMID: 25106862 Review.
-
Strategies for Inhibition of Biofilm Formation on Silicone Rubber Voice Prostheses: A Systematic Review.J Voice. 2023 Aug 23:S0892-1997(23)00222-9. doi: 10.1016/j.jvoice.2023.07.015. Online ahead of print. J Voice. 2023. PMID: 37625903 Review.
Cited by
-
Milieu matters: An in vitro wound milieu to recapitulate key features of, and probe new insights into, mixed-species bacterial biofilms.Biofilm. 2021 Apr 3;3:100047. doi: 10.1016/j.bioflm.2021.100047. eCollection 2021 Dec. Biofilm. 2021. PMID: 33912828 Free PMC article.
-
Inhibition and Removal of Mature Mixed-Bacteria Biofilms on Voice Prostheses by Sodium Selenite.Infect Drug Resist. 2022 Dec 29;15:7799-7810. doi: 10.2147/IDR.S393434. eCollection 2022. Infect Drug Resist. 2022. PMID: 36600950 Free PMC article.
-
Culture media influences Candida parapsilosis growth, susceptibility, and virulence.Front Cell Infect Microbiol. 2023 Dec 13;13:1323619. doi: 10.3389/fcimb.2023.1323619. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38156315 Free PMC article.
-
Effect of silver sulfadiazine on mature mixed bacterial biofilms on voice prostheses.J Otolaryngol Head Neck Surg. 2023 Nov 21;52(1):74. doi: 10.1186/s40463-023-00672-3. J Otolaryngol Head Neck Surg. 2023. PMID: 37990258 Free PMC article.
-
A new spray-based method for the in-vitro development of dry-surface biofilms.Microbiologyopen. 2023 Feb;12(1):e1330. doi: 10.1002/mbo3.1330. Microbiologyopen. 2023. PMID: 36825879 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

