JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies
- PMID: 29649002
- PMCID: PMC6026004
- DOI: 10.1172/JCI98814
JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies
Abstract
Background: Monogenic IFN-mediated autoinflammatory diseases present in infancy with systemic inflammation, an IFN response gene signature, inflammatory organ damage, and high mortality. We used the JAK inhibitor baricitinib, with IFN-blocking activity in vitro, to ameliorate disease.
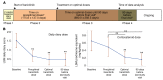
Methods: Between October 2011 and February 2017, 10 patients with CANDLE (chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperatures), 4 patients with SAVI (stimulator of IFN genes-associated [STING-associated] vasculopathy with onset in infancy), and 4 patients with other interferonopathies were enrolled in an expanded access program. The patients underwent dose escalation, and the benefit was assessed by reductions in daily disease symptoms and corticosteroid requirement. Quality of life, organ inflammation, changes in IFN-induced biomarkers, and safety were longitudinally assessed.
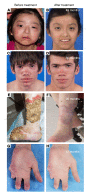
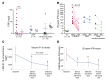
Results: Eighteen patients were treated for a mean duration of 3.0 years (1.5-4.9 years). The median daily symptom score decreased from 1.3 (interquartile range [IQR], 0.93-1.78) to 0.25 (IQR, 0.1-0.63) (P < 0.0001). In 14 patients receiving corticosteroids at baseline, daily prednisone doses decreased from 0.44 mg/kg/day (IQR, 0.31-1.09) to 0.11 mg/kg/day (IQR, 0.02-0.24) (P < 0.01), and 5 of 10 patients with CANDLE achieved lasting clinical remission. The patients' quality of life and height and bone mineral density Z-scores significantly improved, and their IFN biomarkers decreased. Three patients, two of whom had genetically undefined conditions, discontinued treatment because of lack of efficacy, and one CANDLE patient discontinued treatment because of BK viremia and azotemia. The most common adverse events were upper respiratory infections, gastroenteritis, and BK viruria and viremia.
Conclusion: Upon baricitinib treatment, clinical manifestations and inflammatory and IFN biomarkers improved in patients with the monogenic interferonopathies CANDLE, SAVI, and other interferonopathies. Monitoring safety and efficacy is important in benefit-risk assessment.
Trial registration: ClinicalTrials.gov NCT01724580 and NCT02974595.
Funding: This research was supported by the Intramural Research Program of the NIH, NIAID, and NIAMS. Baricitinib was provided by Eli Lilly and Company, which is the sponsor of the expanded access program for this drug.
Keywords: Immunology; Innate immunity; Monogenic diseases; Therapeutics; Translation.
Conflict of interest statement
Figures


Comment in
-
JAK inhibitors in autoinflammation.J Clin Invest. 2018 Jul 2;128(7):2760-2762. doi: 10.1172/JCI121526. Epub 2018 Jun 11. J Clin Invest. 2018. PMID: 29889100 Free PMC article.
Similar articles
-
Pharmacokinetics, Pharmacodynamics, and Proposed Dosing of the Oral JAK1 and JAK2 Inhibitor Baricitinib in Pediatric and Young Adult CANDLE and SAVI Patients.Clin Pharmacol Ther. 2018 Aug;104(2):364-373. doi: 10.1002/cpt.936. Epub 2017 Dec 8. Clin Pharmacol Ther. 2018. PMID: 29134648 Free PMC article.
-
Efficacy and safety of baricitinib in Japanese patients with autoinflammatory type I interferonopathies (NNS/CANDLE, SAVI, And AGS).Pediatr Rheumatol Online J. 2023 Apr 22;21(1):38. doi: 10.1186/s12969-023-00817-8. Pediatr Rheumatol Online J. 2023. PMID: 37087470 Free PMC article. Clinical Trial.
-
Disease flares with baricitinib dose reductions and development of flare criteria in patients with CANDLE/PRAAS.Ann Rheum Dis. 2024 Aug 27;83(9):1181-1188. doi: 10.1136/ard-2023-225463. Ann Rheum Dis. 2024. PMID: 38653530 Free PMC article.
-
Efficacy and Safety of Janus Kinase Inhibitors in Type I Interferon-Mediated Monogenic Autoinflammatory Disorders: A Scoping Review.Dermatol Ther (Heidelb). 2021 Jun;11(3):733-750. doi: 10.1007/s13555-021-00517-9. Epub 2021 Apr 15. Dermatol Ther (Heidelb). 2021. PMID: 33856640 Free PMC article. Review.
-
Baricitinib: therapeutic potential for moderate to severe atopic dermatitis.Expert Opin Investig Drugs. 2020 Oct;29(10):1089-1098. doi: 10.1080/13543784.2020.1800639. Epub 2020 Aug 14. Expert Opin Investig Drugs. 2020. PMID: 32703039 Review.
Cited by
-
The Third Man: DNA sensing as espionage in pulmonary vascular health and disease.Pulm Circ. 2021 Mar 2;11(1):2045894021996574. doi: 10.1177/2045894021996574. eCollection 2021 Jan-Mar. Pulm Circ. 2021. PMID: 33738095 Free PMC article. Review.
-
Current Therapeutic Options for the Main Monogenic Autoinflammatory Diseases and PFAPA Syndrome: Evidence-Based Approach and Proposal of a Practical Guide.Front Immunol. 2020 Jun 3;11:865. doi: 10.3389/fimmu.2020.00865. eCollection 2020. Front Immunol. 2020. PMID: 32655539 Free PMC article. Review.
-
Profiles of host immune impairment in Plasmodium and SARS-CoV-2 infections.Heliyon. 2022 Dec;8(12):e11744. doi: 10.1016/j.heliyon.2022.e11744. Epub 2022 Nov 18. Heliyon. 2022. PMID: 36415655 Free PMC article. Review.
-
Aberrant RNA sensing in regulatory T cells causes systemic autoimmunity.Sci Adv. 2024 Mar;10(9):eadk0820. doi: 10.1126/sciadv.adk0820. Epub 2024 Mar 1. Sci Adv. 2024. PMID: 38427731 Free PMC article.
-
Blockade of the CXCR3/CXCL10 axis ameliorates inflammation caused by immunoproteasome dysfunction.JCI Insight. 2022 Apr 8;7(7):e152681. doi: 10.1172/jci.insight.152681. JCI Insight. 2022. PMID: 35393946 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

