Curcumin as a Promising Antibacterial Agent: Effects on Metabolism and Biofilm Formation in S. mutans
- PMID: 29682545
- PMCID: PMC5851298
- DOI: 10.1155/2018/4508709
Curcumin as a Promising Antibacterial Agent: Effects on Metabolism and Biofilm Formation in S. mutans
Abstract
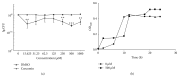
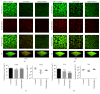
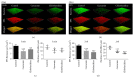
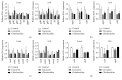
Streptococcus mutans (S. mutans) has been proved to be the main aetiological factor in dental caries. Curcumin, a natural product, has been shown to exhibit therapeutic antibacterial activity, suggesting that curcumin may be of clinical interest. The objective of this study is to evaluate the inhibitory effects of curcumin on metabolism and biofilm formation in S. mutans using a vitro biofilm model in an artificial oral environment. S. mutans biofilms were treated with varying concentrations of curcumin. The biofilm metabolism and biofilm biomass were assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay and the crystal violet assay. Confocal laser scanning microscopy was used to analyse the composition and extracellular polysaccharide content of S. mutans biofilm after curcumin treatment. The biofilm structure was evaluated using a scanning electron microscope. The gene expression of virulence-related factors was assessed by real-time PCR. The antibiofilm effect of curcumin was compared with that of chlorhexidine. The sessile minimum inhibitory concentration (SMIC50%) of curcumin against S. mutans biofilm was 500 μM. Curcumin reduced the biofilm metabolism from 5 min to 24 h. Curcumin inhibited the quantity of live bacteria and total bacteria in both the short term (5 min) and the long term. Moreover, curcumin decreased the production of extracellular polysaccharide in the short term. The expression of genes related to extracellular polysaccharide synthesis, carbohydrate metabolism, adherence, and the two-component transduction system decreased after curcumin treatment. The chlorhexidine-treated group showed similar results. We speculate that curcumin has the capacity to be developed as an alternative agent with the potential to reduce the pathogenic traits of S. mutans biofilm.
Figures


Similar articles
-
The enhancing antibiofilm activity of curcumin on Streptococcus mutans strains from severe early childhood caries.BMC Microbiol. 2020 Sep 16;20(1):286. doi: 10.1186/s12866-020-01975-5. BMC Microbiol. 2020. PMID: 32938379 Free PMC article.
-
Effect of Rubusoside, a Natural Sucrose Substitute, on Streptococcus mutans Biofilm Cariogenic Potential and Virulence Gene Expression In Vitro.Appl Environ Microbiol. 2020 Aug 3;86(16):e01012-20. doi: 10.1128/AEM.01012-20. Print 2020 Aug 3. Appl Environ Microbiol. 2020. PMID: 32503907 Free PMC article.
-
Rhein: A novel antibacterial compound against Streptococcus mutans infection.Microbiol Res. 2022 Aug;261:127062. doi: 10.1016/j.micres.2022.127062. Epub 2022 May 7. Microbiol Res. 2022. PMID: 35597077
-
Antibacterial effects of natural compounds on biofilm formation of Streptococcus mutans.Clin Exp Dent Res. 2022 Dec;8(6):1426-1433. doi: 10.1002/cre2.673. Epub 2022 Oct 25. Clin Exp Dent Res. 2022. PMID: 36281582 Free PMC article. Review.
-
Inhibition of Streptococcus mutans biofilm formation by strategies _targeting the metabolism of exopolysaccharides.Crit Rev Microbiol. 2021 Sep;47(5):667-677. doi: 10.1080/1040841X.2021.1915959. Epub 2021 May 3. Crit Rev Microbiol. 2021. PMID: 33938347 Review.
Cited by
-
Antimicrobial photodynamic therapy (aPDT) for biofilm treatments. Possible synergy between aPDT and pulsed electric fields.Virulence. 2021 Dec;12(1):2247-2272. doi: 10.1080/21505594.2021.1960105. Virulence. 2021. PMID: 34496717 Free PMC article. Review.
-
Strategies for dispersion of cariogenic biofilms: applications and mechanisms.Front Microbiol. 2022 Sep 2;13:981203. doi: 10.3389/fmicb.2022.981203. eCollection 2022. Front Microbiol. 2022. PMID: 36134140 Free PMC article. Review.
-
Antimicrobial and Antibiofilm Activity of Curcumin-Loaded Electrospun Nanofibers for the Prevention of the Biofilm-Associated Infections.Molecules. 2021 Aug 11;26(16):4866. doi: 10.3390/molecules26164866. Molecules. 2021. PMID: 34443457 Free PMC article.
-
Does Curcumin Have an Anticaries Effect? A Systematic Review of In Vitro Studies.Adv Exp Med Biol. 2021;1291:213-227. doi: 10.1007/978-3-030-56153-6_12. Adv Exp Med Biol. 2021. PMID: 34331692
-
Flavonoid Baicalein Suppresses Oral Biofilms and Protects Enamel Hardness to Combat Dental Caries.Int J Mol Sci. 2022 Sep 13;23(18):10593. doi: 10.3390/ijms231810593. Int J Mol Sci. 2022. PMID: 36142516 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

