Liver fat imaging-a clinical overview of ultrasound, CT, and MR imaging
- PMID: 29722568
- PMCID: PMC6223150
- DOI: 10.1259/bjr.20170959
Liver fat imaging-a clinical overview of ultrasound, CT, and MR imaging
Abstract
Hepatic steatosis is a frequently encountered imaging finding that may indicate chronic liver disease, the most common of which is non-alcoholic fatty liver disease. Non-alcoholic fatty liver disease is implicated in the development of systemic diseases and its progressive phenotype, non-alcoholic steatohepatitis, leads to increased liver-specific morbidity and mortality. With the rising obesity epidemic and advent of novel therapeutics aimed at altering metabolism, there is a growing need to quantify and monitor liver steatosis. Imaging methods for assessing steatosis range from simple and qualitative to complex and highly accurate metrics. Ultrasound may be appropriate in some clinical instances as a screening modality to identify the presence of abnormal liver morphology. However, it lacks sufficient specificity and sensitivity to constitute a diagnostic modality for instigating and monitoring therapy. Newer ultrasound techniques such as quantitative ultrasound show promise in turning qualitative assessment of steatosis on conventional ultrasound into quantitative measurements. Conventional unenhanced CT is capable of detecting and quantifying moderate to severe steatosis but is inaccurate at diagnosing mild steatosis and involves the use of radiation. Newer CT techniques, like dual energy CT, show potential in expanding the role of CT in quantifying steatosis. MRI proton-density fat fraction is currently the most accurate and precise imaging biomarker to quantify liver steatosis. As such, proton-density fat fraction is the most appropriate noninvasive end point for steatosis reduction in clinical trials and therapy response assessment.
Figures
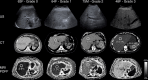
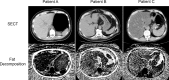
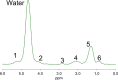

Similar articles
-
Quantitative ultrasound approaches for diagnosis and monitoring hepatic steatosis in nonalcoholic fatty liver disease.Theranostics. 2020 Mar 4;10(9):4277-4289. doi: 10.7150/thno.40249. eCollection 2020. Theranostics. 2020. PMID: 32226553 Free PMC article. Review.
-
Imaging evaluation of non-alcoholic fatty liver disease: focused on quantification.Clin Mol Hepatol. 2017 Dec;23(4):290-301. doi: 10.3350/cmh.2017.0042. Epub 2017 Oct 10. Clin Mol Hepatol. 2017. PMID: 28994271 Free PMC article. Review.
-
Imaging of nonalcoholic fatty liver disease and its clinical utility.Hormones (Athens). 2018 Mar;17(1):69-81. doi: 10.1007/s42000-018-0012-x. Epub 2018 Apr 10. Hormones (Athens). 2018. PMID: 29858854 Review.
-
Quantitative assessment of liver steatosis using ultrasound: dual-energy CT.J Med Ultrason (2001). 2021 Oct;48(4):507-514. doi: 10.1007/s10396-021-01136-9. Epub 2021 Sep 18. J Med Ultrason (2001). 2021. PMID: 34536163 Review.
-
Quantitative Evaluation of Hepatic Steatosis Using Advanced Imaging Techniques: Focusing on New Quantitative Ultrasound Techniques.Korean J Radiol. 2022 Jan;23(1):13-29. doi: 10.3348/kjr.2021.0112. Korean J Radiol. 2022. PMID: 34983091 Free PMC article. Review.
Cited by
-
Quantitative multiparametric MRI allows safe surgical planning in patients undergoing liver resection for colorectal liver metastases: report of two patients.BJR Case Rep. 2021 Jan 12;7(3):20200172. doi: 10.1259/bjrcr.20200172. eCollection 2021 May 1. BJR Case Rep. 2021. PMID: 34131498 Free PMC article.
-
Dual-layer spectral-detector CT for detecting liver steatosis by using proton density fat fraction as reference.Insights Imaging. 2024 Aug 15;15(1):210. doi: 10.1186/s13244-024-01716-6. Insights Imaging. 2024. PMID: 39145877 Free PMC article.
-
Maternal gestational hypertension, smoking and pre-eclampsia are associated with metabolic dysfunction-associated fatty liver disease in overweight offspring.Acta Obstet Gynecol Scand. 2024 Jun;103(6):1183-1191. doi: 10.1111/aogs.14816. Epub 2024 Mar 3. Acta Obstet Gynecol Scand. 2024. PMID: 38433535 Free PMC article.
-
Role of Soluble Adiponectin Receptor 2 in Non-Alcoholic Fatty Liver Disease in Children.Pediatr Gastroenterol Hepatol Nutr. 2019 Sep;22(5):470-478. doi: 10.5223/pghn.2019.22.5.470. Epub 2019 Sep 11. Pediatr Gastroenterol Hepatol Nutr. 2019. PMID: 31555572 Free PMC article.
-
Lipid accumulation product independently correlate with hepatic steatosis quantified by controlled attenuation parameter in women with polycystic ovary syndrome.Endocr Connect. 2020 Feb;9(2):154-162. doi: 10.1530/EC-19-0559. Endocr Connect. 2020. PMID: 31910158 Free PMC article.
References
-
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. . . The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol 2012; 107: 811–26. doi: 10.1038/ajg.2012.128 - DOI - PubMed
-
- Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. . Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011; 140: 124–31. doi: 10.1053/j.gastro.2010.09.038 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

