Overexpression of ATP citrate lyase in renal cell carcinoma tissues and its effect on the human renal carcinoma cells in vitro
- PMID: 29725424
- PMCID: PMC5920499
- DOI: 10.3892/ol.2018.8211
Overexpression of ATP citrate lyase in renal cell carcinoma tissues and its effect on the human renal carcinoma cells in vitro
Abstract
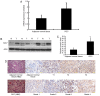
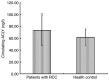
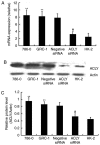
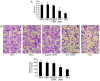
ATP citrate lyase (ACLY) is a key enzyme of lipogenesis in cells. However, ACLY expression in renal cell carcinoma (RCC) and its association with clinicopathological parameters remain unclear. The present study aimed to evaluate ACLY expression levels in RCC and adjacent normal tissues. This study included 33 patients with clear cell RCC (ccRCC). ACLY protein was assayed using immunohistochemistry and western blotting methods. ACLY mRNA expression was determined by reverse transcription-quantitative polymerase chain reaction. Serum ACLY concentrations were measured using the ELISA. Compared with adjacent normal tissues, significantly higher levels of ACLY protein expression were observed in all of the ccRCC tissues (P<0.05). ACLY protein levels were positively associated with the T stage and nuclear grade of RCC. ACLY immunostaining was located in the cytoplasm and nucleus. ACLY protein levels and ACC1 mRNA expression in RCC tissues were significantly higher compared with that in adjacent normal tissues (P<0.05). There were no significant differences in serum ACLY concentrations between patients with RCC and health controls (P>0.05). Preliminary evaluation of ACLY function showed that ACLY small interfering RNA downregulation inhibited RCC cell proliferation and migration, but promoted RCC cell apoptosis. ACLY may be a novel biomarker for the evaluation of biological aggressiveness and may be a potential _target for RCC treatment.
Keywords: ATP citrate lyase; lipogenesis; metabolic; renal cell carcinoma.
Figures
Similar articles
-
The prognostic value and its relationship with immune infiltration of ACLY in clear cell renal cell carcinoma.Transl Oncol. 2024 Sep;47:102056. doi: 10.1016/j.tranon.2024.102056. Epub 2024 Jul 5. Transl Oncol. 2024. PMID: 38970915 Free PMC article.
-
Downregulation of the long noncoding RNA TUG1 inhibits the proliferation, migration, invasion and promotes apoptosis of renal cell carcinoma.J Mol Histol. 2016 Aug;47(4):421-8. doi: 10.1007/s10735-016-9683-2. Epub 2016 Jun 20. J Mol Histol. 2016. PMID: 27323757
-
ATP citrate lyase expression is associated with advanced stage and prognosis in gastric adenocarcinoma.Int J Clin Exp Med. 2015 May 15;8(5):7855-60. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26221340 Free PMC article.
-
ATP citrate lyase (ACLY): a promising _target for cancer prevention and treatment.Curr Drug _targets. 2015;16(2):156-63. doi: 10.2174/1389450115666141224125117. Curr Drug _targets. 2015. PMID: 25537655 Review.
-
ATP citrate lyase (ACLY) inhibitors: An anti-cancer strategy at the crossroads of glucose and lipid metabolism.Eur J Med Chem. 2018 Sep 5;157:1276-1291. doi: 10.1016/j.ejmech.2018.09.001. Epub 2018 Sep 1. Eur J Med Chem. 2018. PMID: 30195238 Review.
Cited by
-
Fatty acid metabolism reprogramming in ccRCC: mechanisms and potential _targets.Nat Rev Urol. 2023 Jan;20(1):48-60. doi: 10.1038/s41585-022-00654-6. Epub 2022 Oct 3. Nat Rev Urol. 2023. PMID: 36192502 Free PMC article. Review.
-
The prognostic value and its relationship with immune infiltration of ACLY in clear cell renal cell carcinoma.Transl Oncol. 2024 Sep;47:102056. doi: 10.1016/j.tranon.2024.102056. Epub 2024 Jul 5. Transl Oncol. 2024. PMID: 38970915 Free PMC article.
-
Metabolite Exchange between Mammalian Organs Quantified in Pigs.Cell Metab. 2019 Sep 3;30(3):594-606.e3. doi: 10.1016/j.cmet.2019.06.002. Epub 2019 Jun 27. Cell Metab. 2019. PMID: 31257152 Free PMC article.
-
The vital role of ATP citrate lyase in chronic diseases.J Mol Med (Berl). 2020 Jan;98(1):71-95. doi: 10.1007/s00109-019-01863-0. Epub 2019 Dec 19. J Mol Med (Berl). 2020. PMID: 31858156 Review.
-
Proteomic analysis of machine perfusion solution from brain dead donor kidneys reveals that elevated complement, cytoskeleton and lipid metabolism proteins are associated with 1-year outcome.Transpl Int. 2021 Sep;34(9):1618-1629. doi: 10.1111/tri.13984. Transpl Int. 2021. PMID: 34448265 Free PMC article.
References
-
- Patel C, Ahmed A, Ellsworth P. Renal cell carcinoma: A reappraisal. Urol Nurs. 2012;32:182–190. - PubMed
-
- Zisman A, Pantuck AJ, Wieder J, Chao DH, Dorey F, Said JW, deKernion JB, Figlin RA, Belldegrun AS. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20:4559–4566. doi: 10.1200/JCO.2002.05.111. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
