NLRP3 regulates platelet integrin αIIbβ3 outside-in signaling, hemostasis and arterial thrombosis
- PMID: 29794149
- PMCID: PMC6119128
- DOI: 10.3324/haematol.2018.191700
NLRP3 regulates platelet integrin αIIbβ3 outside-in signaling, hemostasis and arterial thrombosis
Abstract
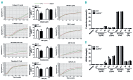
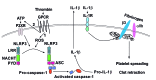
In addition to their hemostatic function, platelets play an important role in regulating the inflammatory response. The platelet NLRP3 inflammasome not only promotes interleukin-1β secretion, but was also found to be upregulated during platelet activation and thrombus formation in vitro However, the role of NLRP3 in platelet function and thrombus formation in vivo remains unclear. In this study, we aimed to investigate the role of NLRP3 in platelet integrin αIIbβ3 signaling transduction. Using NLRP3-/- mice, we showed that NLRP3-deficient platelets do not have significant differences in expression of the platelet-specific adhesive receptors αIIbβ3 integrin, GPIba or GPVI; however, NLRP3-/- platelets transfused into wild-type mice resulted in prolonged tail-bleeding time and delayed arterial thrombus formation, as well as exhibiting impaired spreading on immobilized fibrinogen and defective clot retraction, concomitant with decreased phosphorylation of c-Src, Syk and PLCγ2 in response to thrombin stimulation. Interestingly, addition of exogenous recombinant interleukin-1β reversed the defect in NLRP3-/- platelet spreading and clot retraction, and restored thrombin-induced phosphorylation of c-Src/Syk/PLCγ2, whereas an anti-interleukin-1β antibody blocked spreading and clot retraction mediated by wild-type platelets. Using the direct NLRP3 inhibitor, CY-09, we demonstrated significantly reduced human platelet aggregation in response to threshold concentrations of collagen and ADP, as well as impaired clot retraction in CY-09-treated human platelets, supporting a role for NLRP3 also in regulating human platelet αIIbβ3 outside-in signaling. This study identifies a novel role for NLRP3 and interleukin-1β in platelet function, and provides a new potential link between thrombosis and inflammation, suggesting that therapies _targeting NLRP3 or interleukin-1β might be beneficial for treating inflammation-associated thrombosis.
Copyright© 2018 Ferrata Storti Foundation.
Figures




Similar articles
-
The Antithrombotic Agent Pterostilbene Interferes with Integrin αIIbβ3-Mediated Inside-Out and Outside-In Signals in Human Platelets.Int J Mol Sci. 2021 Mar 31;22(7):3643. doi: 10.3390/ijms22073643. Int J Mol Sci. 2021. PMID: 33807403 Free PMC article.
-
PPARγ agonists negatively regulate αIIbβ3 integrin outside-in signaling and platelet function through up-regulation of protein kinase A activity.J Thromb Haemost. 2017 Feb;15(2):356-369. doi: 10.1111/jth.13578. Epub 2017 Feb 7. J Thromb Haemost. 2017. PMID: 27896950 Free PMC article.
-
Platycodin D inhibits platelet function and thrombus formation through inducing internalization of platelet glycoprotein receptors.J Transl Med. 2018 Nov 15;16(1):311. doi: 10.1186/s12967-018-1688-z. J Transl Med. 2018. PMID: 30442147 Free PMC article.
-
Integrin αIIbβ3 outside-in signaling.Blood. 2017 Oct 5;130(14):1607-1619. doi: 10.1182/blood-2017-03-773614. Epub 2017 Aug 9. Blood. 2017. PMID: 28794070 Free PMC article. Review.
-
Beta3 tyrosine phosphorylation in alphaIIbbeta3 (platelet membrane GP IIb-IIIa) outside-in integrin signaling.Thromb Haemost. 2001 Jul;86(1):246-58. Thromb Haemost. 2001. PMID: 11487013 Review.
Cited by
-
Protective Role of Platelets in Myocardial Infarction and Ischemia/Reperfusion Injury.Cardiol Res Pract. 2021 May 24;2021:5545416. doi: 10.1155/2021/5545416. eCollection 2021. Cardiol Res Pract. 2021. PMID: 34123416 Free PMC article. Review.
-
Neutrophil extracellular traps and inflammasomes cooperatively promote venous thrombosis in mice.Blood Adv. 2021 May 11;5(9):2319-2324. doi: 10.1182/bloodadvances.2020003377. Blood Adv. 2021. PMID: 33938940 Free PMC article.
-
TLR4-dependent upregulation of the platelet NLRP3 inflammasome promotes platelet aggregation in a murine model of hindlimb ischemia.Biochem Biophys Res Commun. 2019 Jan 8;508(2):614-619. doi: 10.1016/j.bbrc.2018.11.125. Epub 2018 Dec 3. Biochem Biophys Res Commun. 2019. PMID: 30522866 Free PMC article.
-
Mechanisms of NLRP3 Inflammasome Activation: Its Role in the Treatment of Alzheimer's Disease.Neurochem Res. 2020 Nov;45(11):2560-2572. doi: 10.1007/s11064-020-03121-z. Epub 2020 Sep 14. Neurochem Res. 2020. PMID: 32929691 Review.
-
Supplementation with omega-3 or omega-6 fatty acids attenuates platelet reactivity in postmenopausal women.Clin Transl Sci. 2022 Oct;15(10):2378-2391. doi: 10.1111/cts.13366. Epub 2022 Jul 25. Clin Transl Sci. 2022. PMID: 35791734 Free PMC article.
References
-
- Qiao JL, Shen Y, Gardiner EE, Andrews RK. Proteolysis of platelet receptors in humans and other species. Biol Chem. 2010;391(8):893–900. - PubMed
-
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25. - PubMed
-
- Clark EA, Shattil SJ, Ginsberg MH, Bolen J, Brugge JS. Regulation of the protein tyrosine kinase pp72syk by platelet agonists and the integrin alpha IIb beta 3. J Biol Chem. 1994;269(46):28859–28864. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

