Light sheet approaches for improved precision in 3D localization-based super-resolution imaging in mammalian cells [Invited]
- PMID: 29801343
- PMCID: PMC6005674
- DOI: 10.1364/OE.26.013122
Light sheet approaches for improved precision in 3D localization-based super-resolution imaging in mammalian cells [Invited]
Abstract
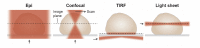
The development of imaging techniques beyond the diffraction limit has paved the way for detailed studies of nanostructures and molecular mechanisms in biological systems. Imaging thicker samples, such as mammalian cells and tissue, in all three dimensions, is challenging due to increased background and volumes to image. Light sheet illumination is a method that allows for selective irradiation of the image plane, and its inherent optical sectioning capability allows for imaging of biological samples with reduced background, photobleaching, and photodamage. In this review, we discuss the advantage of combining single-molecule imaging with light sheet illumination. We begin by describing the principles of single-molecule localization microscopy and of light sheet illumination. Finally, we present examples of designs that successfully have married single-molecule super-resolution imaging with light sheet illumination for improved precision in mammalian cells.
Figures
Similar articles
-
Viewpoint: Localization Microscopy of Single Molecules Enhanced by 3D Imaging and Light Sheet Illumination.J Phys D Appl Phys. 2019 Jan 2;52(1):011001. doi: 10.1088/1361-6463/aae632. Epub 2018 Oct 24. J Phys D Appl Phys. 2019. PMID: 31160828 Free PMC article.
-
Light Sheet Illumination for 3D Single-Molecule Super-Resolution Imaging of Neuronal Synapses.Front Synaptic Neurosci. 2021 Nov 24;13:761530. doi: 10.3389/fnsyn.2021.761530. eCollection 2021. Front Synaptic Neurosci. 2021. PMID: 34899261 Free PMC article. Review.
-
Virtual-'light-sheet' single-molecule localisation microscopy enables quantitative optical sectioning for super-resolution imaging.PLoS One. 2015 Apr 17;10(4):e0125438. doi: 10.1371/journal.pone.0125438. eCollection 2015. PLoS One. 2015. PMID: 25884495 Free PMC article.
-
Resolution doubling in light-sheet microscopy via oblique plane structured illumination.Nat Methods. 2022 Nov;19(11):1419-1426. doi: 10.1038/s41592-022-01635-8. Epub 2022 Oct 24. Nat Methods. 2022. PMID: 36280718 Free PMC article.
-
Light sheet microscopy and live imaging of plants.J Microsc. 2016 Aug;263(2):158-64. doi: 10.1111/jmi.12393. Epub 2016 Mar 28. J Microsc. 2016. PMID: 27019306 Review.
Cited by
-
Viewpoint: Localization Microscopy of Single Molecules Enhanced by 3D Imaging and Light Sheet Illumination.J Phys D Appl Phys. 2019 Jan 2;52(1):011001. doi: 10.1088/1361-6463/aae632. Epub 2018 Oct 24. J Phys D Appl Phys. 2019. PMID: 31160828 Free PMC article.
-
Frontiers in artificial intelligence-directed light-sheet microscopy for uncovering biological phenomena and multi-organ imaging.View (Beijing). 2024 Oct;5(5):20230087. doi: 10.1002/VIW.20230087. Epub 2024 Sep 3. View (Beijing). 2024. PMID: 39478956
-
Whole-cell multi-_target single-molecule super-resolution imaging in 3D with microfluidics and a single-objective tilted light sheet.bioRxiv [Preprint]. 2024 Sep 16:2023.09.27.559876. doi: 10.1101/2023.09.27.559876. bioRxiv. 2024. Update in: Nat Commun. 2024 Nov 24;15(1):10187. doi: 10.1038/s41467-024-54609-z PMID: 37808751 Free PMC article. Updated. Preprint.
-
Instantaneous non-diffracting light-sheet generation by controlling spatial coherence.Opt Commun. 2020 Nov 1;474:126154. doi: 10.1016/j.optcom.2020.126154. Epub 2020 May 30. Opt Commun. 2020. PMID: 34483370 Free PMC article.
-
SOLEIL: single-objective lens inclined light sheet localization microscopy.Biomed Opt Express. 2022 May 10;13(6):3275-3294. doi: 10.1364/BOE.451634. eCollection 2022 Jun 1. Biomed Opt Express. 2022. PMID: 35781973 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

